Choi and Park: The Incidence and Characteristics of Patients with Small Ruptured Aneurysms (<5 mm) in Subarachnoid Hemorrhage
Abstract
Objective
Small unruptured aneurysms (<5 mm) are known for their very low risk of rupture, and are recommended to be treated conservatively. However, we encounter many patients with small ruptured aneurysms in the clinical practice. We aimed to investigate the incidence and characteristics of patients with small ruptured aneurysms.
Methods
We reviewed all patients admitted to our hospital with subarachnoid hemorrhage from January 2005 to December 2015. The patients were divided into two groups: those with aneurysms <5 mm (group S) and those with aneurysms ≥5 mm (group L). The patient’s age and sex, size and location of aneurysms, and risk factors such as hypertension, diabetes, alcohol use, and smoking were compared between the two groups.
Results
Eight-hundred eleven patients were diagnosed with ruptured aneurysms, and 337 (41.6%) were included in group S. The mean size of all aneurysms was 6.10±2.99 mm (range, 0.7-37.7); aneurysms with a diameter of 4-5 mm accounted for the largest subgroup of all aneurysms. Female sex was significantly associated with the incidence of small ruptured aneurysms (odds ratio [OR] 1.50, 95% confidence intervals [CI] 1.02-2.19, p=0.037). Despite female predominance in the incidence of small ruptured aneurysms, the proportion of small ruptured aneurysms in young (<50 years) men was high. In men, there were no significant differences regarding the location of the aneurysms between group S and group L (p=0.267), with the most frequent location being the anterior communicating artery (ACoA) in both group S (50.9%) and group L (51.4%). However, in women, there were significant differences regarding the location of the aneurysms between group S and group L (p=0.023), with the most frequent locations being the ACoA (33.0%) in group S, and the posterior communicating artery (30.6%) in group L. In women, two locations were significantly associated with small (<5 mm) ruptured aneurysms: the ACoA (OR 2.14, 95% CI 1.01-4.54, p=0.047) and anterior cerebral artery (OR 3.54, 95% CI 1.19-10.54, p=0.023). Multiplicity and smoking were significantly associated with large (≥5 mm) ruptured aneurysms in women. The use of alcohol was related to small ruptured aneurysms in men over 50 years of age (OR 2.23, 95% CI 1.03-4.84, p=0.042).
Conclusion
In this study, small (<5 mm) ruptured aneurysms exhibited different incidences by age, sex, location, and risk factors such as multiplicity, smoking, and alcohol use.
Key Words: Subarachnoid hemorrhage · Intracranial aneurysm · Rupture · Risk factors.
INTRODUCTION
Intracranial aneurysms are relatively common lesions, with a prevalence of approximately 5% 23). Recently, unruptured aneurysms are increasingly detected due to the increased availability and improved sensitivity of noninvasive imaging techniques 14). The most common presentation of intracranial aneurysm is rupture, which leads to subarachnoid hemorrhage (SAH). Previous studies reported that overall mortality rates of aneurysmal SAH range between 32% and 67%, and about 30% of survivors exhibit moderate to severe disability 5). The size of the intracranial aneurysm is an important risk factor for rupture, and the International Study of Unruptured Intracranial Aneurysms (ISUIA) reported much lower 5-year cumulative rupture rates for small aneurysms 7). It is currently recommended that small, incidental aneurysms measuring less than 5 mm in diameter be managed conservatively 8,9,15,28). However, we encounter many cases of the rupture of small aneurysms measuring less than 5 mm in clinical practice. Therefore, the main purpose of this study was to investigate the incidence and characteristics of patients with small (<5 mm) ruptured aneurysms.
MATERIALS AND METHODS
Nine hundred six patients were admitted at our hospital with SAH between January 2005 and December 2015. A total of 811 patients were included in this study ( Table 1), after excluding 95 cases of 21 patients with a dissecting aneurysm, 47 patients with SAH of unknown origin, and 27 patients for whom no angiographic data were available. Various factors retrieved from medical records and radiological findings were analyzed, including the size and location of aneurysms, as well as associated risk factors including hypertension, diabetes mellitus, aneurysm multiplicity, alcohol use, and smoking. Multiplicity of aneurysm was defined in this study as the occurrence of more than two aneurysms. Alcohol use was defined as having up to 15 drinks or more per week and smoking was defined as current smokers and ex-smokers. Digital subtraction angiography was used for measuring the size of aneurysms. We defined the size of an aneurysm as the largest diameter measured based on the long axis of the aneurysm. In multiple aneurysms, the ruptured aneurysm was confirmed by the hemorrhage distribution on computed tomography scan, size, and morphology. The aneurysm sizes were divided into two groups: those smaller than 5 mm in diameter (group S) and those larger than or equal to 5 mm (group L). The location of the aneurysm was classified as follows: 1) anterior communicating artery (ACoA), 2) posterior communicating artery (PCoA), 3) internal carotid artery (ICA), 4) middle cerebral artery (MCA), 5) anterior cerebral artery (ACA), and 6) posterior circulation, including the posterior cerebral artery, basilar artery, and vertebral artery.
Statistical analysis was performed using SPSS software (SPSS version 21.0; SPSS Inc., Chicago, IL, USA). The chi-square test and t-test were used for comparisons between group S and group L, as appropriate. Odds ratio (OR) for comparison of the two groups were summarized with 95% confidence intervals (CI) and p values using logistic regression analysis. p values lower than 0.05 were considered statistically significant.
RESULTS
Distribution of ruptured aneurysms according to age and sex
The mean age of all patients was 60.33±12.73 years (range, 26-96). The mean age of men (54.59±11.13) was significantly lower than that of women (63.47±12.46; p<0.001). The mean age of patients in group S (59.01±12.73) was lower than that of patients in group L (61.27±12.66, p=0.013). Regarding the mean age of women, a statistically significant difference was observed between the two groups (group S, 61.91±11.96 years; group L, 64.67±12.73 years; p=0.012). In men, ruptured aneurysms showed the highest incidence in the 40s and 50s in group S, while they had the highest incidence in the 50s and 60s in group L ( p=0.052) ( Fig. 1A). In women, ruptured aneurysms showed the highest incidence in their 50s and 60s for group S, while they had the highest incidence in their 50s and 70s in group L ( p=0.044) ( Fig. 1B). Small ruptured aneurysms were more prevalent among those with young age (<50 years) in both men and women, and especially those with young age (<40 years) in male patients. Five hundred twenty-four patients were women (64.6%) and 287 were men (35.4%). The proportion of women in group S (67.4%) was larger than that in group L (62.7%). Table 2 shows that female sex is significantly associated with the incidence of small (<5 mm) ruptured aneurysms (OR 1.50, 95% CI 1.02-2.19, p=0.037).
Distribution of ruptured aneurysms according to size and location
Three hundred thirty-seven (41.6%) patients were included in group S. The mean size of all aneurysms was 6.10±2.99 mm (range, 0.7-37.7). After categorizing aneurysms by differences of 1 mm in diameter, aneurysms with a diameter of 4-5 mm accounted for the largest subgroup of aneurysms ( Fig. 2). After comparing by sex, women most commonly exhibited ruptured aneurysms with a diameter of 4-5 mm, and men most commonly exhibited ruptured aneurysms with a diameter of 5-6 mm ( p=0.187). The most frequent location of aneurysms of all size was the ACoA (35.9%), followed by the MCA (27.0%), and then the PCoA (20.5%). In men, there were no significant differences regarding the location of aneurysms between group S and group L ( p=0.267), with the most frequent location being the ACoA in both group S (50.9%) and group L (51.4%). However, in women, there were significant differences regarding the location of aneurysms between group S and group L ( p=0.023), with the most frequent locations being the ACoA (33.0%) in group S and the PCoA (30.6%) in group L. Two locations in women that were significantly associated with small (<5 mm) ruptured aneurysms ( Table 2); ACoA (OR 2.14, 95% CI 1.01-4.54, p=0.047) and ACA (OR 3.54, 95% CI 1.19-10.54, p=0.023).
Risk factors for rupture of aneurysms
Several risk factors including multiplicity of the aneurysms, hypertension, diabetes mellitus, smoking, and alcohol use were evaluated. After evaluating the risk factors, the presence of hypertension or diabetes was not mean to be statistically significant between group S and group L. Multiplicity of the aneurysms and smoking were more frequent in group L than group S. The risk factors of multiplicity of the aneurysms (OR 0.65, 95% CI 0.46-0.93, p=0.019) and smoking (OR 0.32, 95% CI 0.17-0.64, p=0.001) were statistically significant only in women, as seen in Table 2. The use of alcohol was likely related to small (<5 mm) ruptured aneurysms (OR 1.78, 95% CI 0.96-3.30, p=0.066) only in male patients. In patients with small ruptured aneurysms (group S), the use of alcohol was more prevalent in those over 50 years old (66.7%) than in patients under 50 years old (58.1%). In young (<50 years) men, young age was a stronger factor related with small (<5 mm) ruptured aneurysms (OR 2.03, 95% CI 1.21-3.40, p=0.007) than the use of alcohol (OR 1.39, 95% CI 0.85-2.28, p=0.193) on multivariate analysis ( Table 3). Among men over 50 years old, the use of alcohol was significantly related to small ruptured aneurysms (OR 2.23, 95% CI 1.03-4.84, p=0.042) ( Table 4).
DISCUSSION
The size of an intracranial aneurysm is one of the most important criterion when deciding on the treatment for unruptured aneurysms. Aneurysm size, as a criterion influencing treatment decision-making, was decreased from 10 mm in the first ISUIA trial to 7 mm in the second ISUIA trial 7,31), and recent guidelines suggest that unruptured aneurysms less than 5 mm in diameter should be managed conservatively 8,9,15,28). Both ISUIA trials revealed that unruptured aneurysms had a much lower risk of rupture than expected; according to the first ISUIA results, the risk of rupture for unruptured aneurysms with less than 10 mm in diameter was only 0.05% per year in patients with no SAH history 7); the second ISUIA study revealed that the risk of rupture for aneurysms with less than 7 mm in diameter was 0.15% per year in patients with no SAH history 31). However, the extremely low risk of rupture associated with unruptured aneurysms in both ISUIA studies was widely criticized for selection bias, crossover due to switching of therapeutic intervention, and incomplete follow-up. Furthermore, a large portion of the ruptured aneurysms encountered in clinical practice are small in size 1,3,10,16,29). In this study, 41.6% of patients had ruptured aneurysms less than 5 mm, and the largest proportion of aneurysms were between 4 and 5 mm among all aneurysms. Why many patients with SAH and small-sized aneurysms are seen in clinical practice although the rupture rate of small unruptured aneurysms is reported very low? Some authors suggested that the size of the aneurysms may decrease after rupture 32). However, Rahman et al. 22) studied whether cerebral aneurysms shrink with rupture by comparing pre- and post-rupture images of 9 patients with cerebral aneurysms, and they reported that aneurysms do not shrink after rupture. The small unruptured intracranial aneurysm verification study (SUAVe study) also found no shrinkage of aneurysms after rupture in their data 25). Kataoka et al. 13) investigated histological findings for both unruptured and ruptured aneurysms, and found no histological evidence to support the shrinkage of aneurysms after rupture. Yonekura 33) demonstrated that the growth process of an aneurysm from its occurrence can be classified into one of four patterns: type 1, the aneurysm ruptures within a time span as short as a few days to a few weeks after its formation; type 2, the aneurysm grows slowly for a few years after its formation, and then ruptures during this process; type 3, the formed aneurysm continues growing slowly for a few years without rupture; and type 4, the aneurysm grows to a certain size and remains unchanged thereafter. We believe that, in certain patients, cerebral aneurysms rupture easily even when they are small in size and within a relatively shorter period after formation (corresponding to type 1 aneurysms), and this is usually observed as a SAH in clinical practice. On the other hand, most unruptured aneurysms diagnosed incidentally have already passed into the safe period and have a low risk of rupture (corresponding to type 4 aneurysms). These concepts may explain the discrepancy between the very low risk of rupture associated with small unruptured aneurysms and the small size of many ruptured aneurysms. The characteristics of patients with small ruptured aneurysms (corresponding to type 1 aneurysms) may be different from those of patients with large ruptured aneurysms (corresponding to type 2 aneurysms). In this study, small (<5 mm) ruptured aneurysms were significantly associated with female sex ( Table 2). Female sex is a recognized risk factor for the rupture of cerebral aneurysms 27), and aneurysm formation especially in postmenopausal women, where estrogen deficiency has an important impact on the pathophysiology of formation and rupture of cerebral aneurysms 26). This study also revealed the association of small ruptured aneurysms with young male patients, showing a higher incidence between 30 and 40 years of age ( Fig. 1A). Despite a female predominance in small ruptured aneurysms, it was interesting that the incidence of small ruptured aneurysm was high in young men, especially in those aged <40 years. Heavy drinking is more frequently observed among men with SAH than women with SAH 11), and alcohol use is known to increase the risk of SAH 17). In this study, the use of alcohol was significantly related to small ruptured aneurysms in patients over 50 years old. In young (<50 years) men, the statistical analysis revealed that young age was more significantly related with small (<5 mm) ruptured aneurysms than the use of alcohol. In this study, the ACoA was the most common aneurysm site in men, accounting for approximately 50% of both small and large aneurysms. However, in women, small aneurysms were more likely to occur in the ACoA, but large aneurysms were more likely to occur in the PCoA. Silva Neto et al. 24) suggested that the higher frequency of fetal-type PCoA and the smaller angle of the carotid artery might be responsible for the higher prevalence of PCoA aneurysms in women. We found that small and large aneurysms exhibited a similar pattern of location in men. However, there was a different pattern in the location of small and large aneurysms in women; small ruptured aneurysms were significantly associated with the location of the ACoA and ACA. Previous studies found that ruptured aneurysms located in the ACoA and ACA were smaller than those located at other sites 19,30). The size of an aneurysm at the time of rupture may be partly determined by the thickness and diameter of the parent artery 18). Since the diameter of the ACoA and ACA is smaller than that of the MCA and ICA 2), aneurysms located in the ACoA and ACA can rupture before they reach a larger size. With regards to the differences of aneurysm location by sex, anatomical differences in the circle of Willis may cause different hemodynamic stress and thus result in different pathways and mechanism of aneurysm formation 24). In previous papers, it was reported that larger ruptured aneurysms exhibit multiplicity more frequently 6,18,21). We also found that multiplicity was significantly more common in patients with aneurysms larger than 5 mm. Inagawa 6) proposed a hypothesis that may explain our results. When considering the time interval from new development of aneurysms to rupture, in patients with large ruptured aneurysms, newly developed aneurysms do not easily rupture when they are small. Furthermore, the longer the interval from development to rupture, the higher the possibility that an additional aneurysm will develop, resulting in multiple aneurysms, and that the size of the aneurysms will increase 6). In this study, smoking was also significantly more common in the group with aneurysms larger than 5 mm. Smoking has been known to be strongly associated with the growth of cerebral aneurysms 4,12). Smoking was more common in patients with larger aneurysms in a multicenter research trial 21), and Qureshi et al. 20) reported that smoking was associated with multiple aneurysms. These were consistent with our results that multiplicity and smoking were more prevalent in patients with larger aneurysms. The main limitation of our study is its retrospective nature. Smoking and multiplicity were associated with large ruptured aneurysms in this study, which does not indicate that such risk factors are not relevant for the future rupture of small un-ruptured aneurysms. Prospective studies are warranted to investigate the impact of such risk factors on the rupture of small unruptured aneurysms. However, we believe that the following results are clinically meaningful for identifying the characteristics of patients with small ruptured aneurysms: female sex, younger (<50 years) age in men, ACoA or ACA aneurysm location in women, and the use of alcohol in men over 50 years were significantly associated with small ruptured aneurysms. Another limitation of this study is that we did not include the aneurysm shape like a daughter sac, which is also known as a risk factor for aneurysm rupture. The optimal way to study the natural history and risk factors for the rupture of unruptured small aneurysms is simply to observe without treatment. However, such an approach is risky and practically impossible. Further research regarding the factors associated with the rupture of small aneurysms is warranted to gather information to assist in guiding the decision-making process with respect to the best treatment strategy of incidentally identified small aneurysms.
CONCLUSION
In this study, small ruptured aneurysms <5 mm were found in a large portion of about 40% of all SAH patients, and revealed a different distribution among variables including age, sex, location, and risk factors such as multiplicity, alcohol use, and smoking, compared with ruptured aneurysms ≥5 mm. Female sex, young (<50 years) age in men, ACoA and ACA locations in female patients, and the use of alcohol in men over 50 years old were associated with small (<5 mm) ruptured aneurysms. Although aneurysms size is important in the prediction of aneurysm rupture, other various factors should be considered when deciding on the treatment for incidentally identified small aneurysms.
Acknowledgements
This work was supported by the Dong-A University Research Fund.
Fig. 1
Distribution of patients with ruptured aneurysms stratified by age and gender. A: Male patients. B: Female patients. 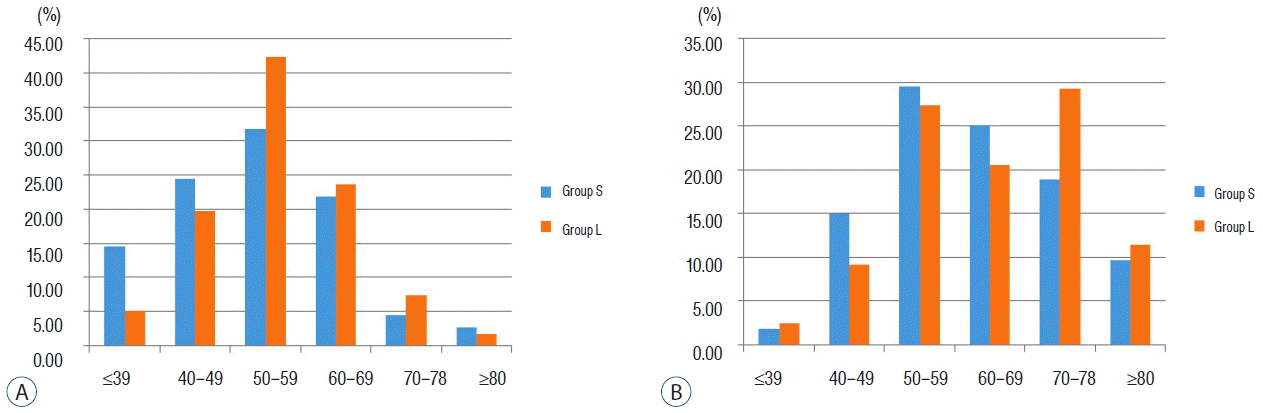
Fig. 2
Distribution of patients with ruptured aneurysms, stratified by size of the aneurysm. 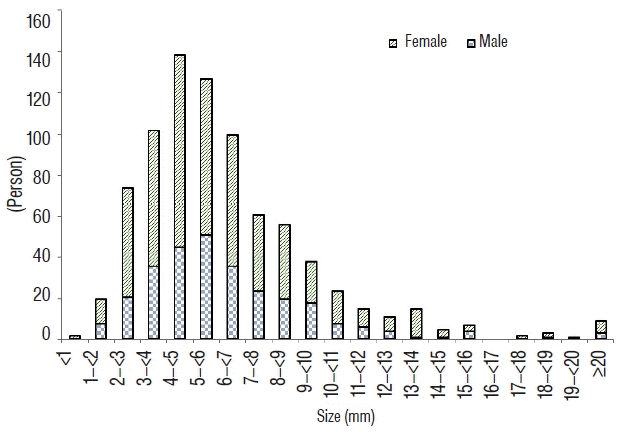
Table 1
Patient information and clinical characteristics
|
Total |
Male patients |
Female patients |
|
|
|
|
Size |
p-value |
Size |
p-value |
Size |
p-value |
|
|
|
|
Total |
Small (<5 mm) |
Large (≥5 mm) |
Small (<5 mm) |
Large (≥5 mm) |
Small (<5 mm) |
Large (≥5 mm) |
|
Total |
811 |
337 (41.6) |
474 (58.4) |
|
110 (38.3) |
177 (61.7) |
|
227 (43.3) |
297 (56.7) |
|
|
|
Sex |
|
Male |
287 (35.4) |
110 (32.6) |
177 (37.3) |
0.168 |
|
|
|
|
|
|
|
Female |
524 (64.6) |
227 (67.4) |
297 (62.7) |
|
|
|
|
|
|
|
|
|
Age (yrs) |
|
Mean±SD |
60.33±12.73 |
59.01±12.73 |
61.27±12.66 |
0.013 |
53.04±12.23 |
55.56±10.30 |
0.062 |
61.91±11.96 |
64.67±12.73 |
0.012 |
|
≤39 |
36 (4.4) |
20 (5.9) |
16 (3.4) |
0.032 |
16 (14.5) |
9 (5.1) |
0.052 |
4 (1.8) |
7 (2.4) |
0.044 |
|
40-49 |
123 (15.2) |
61 (18.1) |
62 (13.1) |
|
27 (24.5) |
35 (19.8) |
|
34 (15.0) |
27 (9.1) |
|
|
50-59 |
258 (31.8) |
102 (30.3) |
156 (32.9) |
|
35 (31.8) |
75 (42.4) |
|
67 (29.5) |
81 (27.3) |
|
|
60-69 |
184 (22.7) |
81 (24.0) |
103 (21.7) |
|
24 (21.8) |
42 (23.7) |
|
57 (25.1) |
61 (20.5) |
|
|
70-79 |
148 (18.2) |
48 (14.2) |
100 (21.1) |
|
5 (4.5) |
13 (7.3) |
|
43 (18.9) |
87 (29.3) |
|
|
≥80 |
62 (7.6) |
25 (7.4) |
37 (7.8) |
|
3 (2.7) |
3 (1.7) |
|
22 (9.7) |
34 (11.4) |
|
|
|
Location |
|
ACoA |
291 (35.9) |
131 (38.9) |
160 (33.8) |
0.193 |
56 (50.9) |
91 (51.4) |
0.267 |
75 (33.0) |
69 (23.2) |
0.023 |
|
PCoA |
166 (20.5) |
59 (17.5) |
107 (22.6) |
|
11 (10.0) |
16 (9.0) |
|
48 (21.1) |
91 (30.6) |
|
|
ICA |
59 (7.3) |
22 (6.5) |
37 (7.8) |
|
8 (7.3) |
9 (5.1) |
|
14 (6.2) |
28 (9.4) |
|
|
MCA |
219 (27.0) |
87 (25.8) |
132 (27.8) |
|
24 (21.8) |
53 (29.9) |
|
63 (27.8) |
79 (26.6) |
|
|
ACA |
31 (3.8) |
17 (5.0) |
14 (3.0) |
|
3 (2.7) |
4 (2.3) |
|
14 (6.2) |
10 (3.4) |
|
|
PC |
45 (5.5) |
21 (6.2) |
24 (5.1) |
|
8 (7.3) |
4 (2.3) |
|
13 (5.7) |
20 (6.7) |
|
|
|
Multiplicity |
|
No |
608 (76.8) |
268 (81.0) |
340 (73.8) |
0.018 |
92 (86.0) |
139 (80.3) |
0.228 |
176 (78.6) |
201 (69.8) |
0.025 |
|
Yes |
184 (23.2) |
63 (19.0) |
121 (26.2) |
|
15 (14.0) |
34 (19.7) |
|
48 (21.4) |
87 (30.2) |
|
|
|
Hypertension |
|
No |
503 (62.0) |
215 (63.8) |
288 (60.8) |
0.380 |
77 (70.0) |
122 (68.9) |
0.848 |
138 (60.8) |
166 (55.9) |
0.260 |
|
Yes |
308 (38.0) |
122 (36.2) |
186 (39.2) |
|
33 (30.0) |
55 (31.1) |
|
89 (39.2) |
131 (44.1) |
|
|
|
Diabetes mellitus |
|
No |
759 (93.6) |
313 (92.9) |
446 (94.1) |
0.487 |
104 (94.5) |
169 (95.5) |
0.721 |
209 (92.1) |
277 (93.3) |
0.601 |
|
Yes |
52 (6.4) |
24 (7.1) |
28 (5.9) |
|
6 (5.5) |
8 (4.5) |
|
18 (7.9) |
20 (6.7) |
|
|
|
Alcohol |
|
No |
565 (69.8) |
232 (69.0) |
333 (70.4) |
0.679 |
40 (36.7) |
78 (44.3) |
0.204 |
192 (84.6) |
255 (85.9) |
0.682 |
|
Yes |
244 (30.2) |
104 (31.0) |
140 (29.6) |
|
69 (63.3) |
98 (55.7) |
|
35 (15.4) |
42 (14.1) |
|
|
|
Smoking |
|
No |
588 (72.6) |
258 (76.8) |
330 (69.6) |
0.024 |
47 (43.1) |
77 (43.5) |
0.949 |
211 (93.0) |
253 (85.2) |
0.006 |
|
Yes |
222 (27.4) |
78 (23.2) |
144 (30.4) |
|
62 (56.9) |
100 (56.5) |
|
|
44 (14.8) |
|
Table 2
Logistic regression analysis of independent contributions of variables for small (<5 mm) ruptured aneurysms
|
Total |
Male patients |
Female patients |
|
|
|
|
OR (95% CI) |
p-value |
OR (95% CI) |
p-value |
OR (95% CI) |
p-value |
|
Sex |
|
Male |
1.00 (reference) |
|
|
|
|
|
|
Female |
1.50 (1.02-2.19) |
0.037 |
|
|
|
|
|
|
Age (yrs) |
|
≤39 |
1.00 (reference) |
|
1.00 (reference) |
|
1.00 (reference) |
|
|
40-49 |
0.65 (0.30-1.43) |
0.288 |
0.37 (0.13-1.03) |
0.058 |
2.09 (0.53-8.26) |
0.295 |
|
50-59 |
0.40 (0.19-0.84) |
0.015 |
0.21 (0.08-0.56) |
0.002 |
1.36 (0.37-5.05) |
0.643 |
|
60-69 |
0.47 (0.22-1.01) |
0.054 |
0.27 (0.10-0.77) |
0.014 |
1.52 (0.40-5.78) |
0.543 |
|
70-79 |
0.31 (0.14-0.69) |
0.004 |
0.21 (0.05-0.85) |
0.028 |
0.94 (0.24-3.64) |
0.931 |
|
≥80 |
0.43 (0.18-1.05) |
0.065 |
0.49 (0.07-3.26) |
0.457 |
1.25 (0.31-5.13) |
0.754 |
|
|
Location |
|
ACoA |
1.45 (0.79-2.66) |
0.228 |
0.50 (0.17-1.53) |
0.226 |
2.14 (1.01-4.54) |
0.047 |
|
PCoA |
0.93 (0.49-1.77) |
0.814 |
0.61 (0.16-2.27) |
0.461 |
1.06 (0.49-2.28) |
0.885 |
|
ICA |
1.00 (reference) |
|
1.00 (reference) |
|
1.00 (reference) |
|
|
MCA |
1.18 (0.63-2.19) |
0.611 |
0.40 (0.12-1.29) |
0.126 |
1.66 (0.78-3.56) |
0.191 |
|
ACA |
2.23 (0.90-5.55) |
0.083 |
0.61 (0.09-4.10) |
0.610 |
3.54 (1.19-10.54) |
0.023 |
|
PC |
1.44 (0.63-3.28) |
0.386 |
1.59 (0.31-8.12) |
0.577 |
1.26 (0.46-3.44) |
0.648 |
|
|
Multiplicity |
|
No |
1.00 (reference) |
|
1.00 (reference) |
|
1.00 (reference) |
|
|
Yes |
0.65 (0.46-0.93) |
0.019 |
0.63 (0.30-1.30) |
0.208 |
0.64 (0.42-0.98) |
0.040 |
|
|
Hypertension |
|
No |
1.00 (reference) |
|
1.00 (reference) |
|
1.00 (reference) |
|
|
Yes |
0.86 (0.62-1.17) |
0.336 |
0.79 (0.44-1.40) |
0.414 |
0.86 (0.58-1.27) |
0.453 |
|
|
Diabetes mellitus |
|
No |
1.00 (reference) |
|
1.00 (reference) |
|
1.00 (reference) |
|
|
Yes |
1.43 (0.79-2.61) |
0.237 |
1.72 (0.54-5.52) |
0.361 |
1.44 (0.70-2.95) |
0.318 |
|
|
Alcohol |
|
No |
1.00 (reference) |
|
1.00 (reference) |
|
1.00 (reference) |
|
|
Yes |
1.39 (0.94-2.07) |
0.101 |
1.78 (0.96-3.30) |
0.066 |
1.38 (0.78-2.42) |
0.270 |
|
|
Smoking |
|
No |
1.00 (reference) |
|
1.00 (reference) |
|
1.00 (reference) |
|
|
Yes |
0.57 (0.38-0.86) |
0.008 |
0.70 (0.38-1.29) |
0.250 |
0.32 (0.16-0.62) |
0.001 |
Table 3
Multivariate analysis of independent contributions of variables for small (<5 mm) ruptured aneurysms in young (<50 years) patients: young age (<50 years) vs. the use of alcohol
|
Male patients |
Female patients |
|
|
|
OR (95% CI) |
p-value |
OR (95% CI) |
p-value |
|
Alcohol |
|
No |
1.00 (reference) |
|
1.00 (reference) |
|
|
Yes |
1.39 (0.85-2.28) |
0.193 |
1.03 (0.63-1.69) |
0.899 |
|
|
Age |
|
Old (≥50) |
1.00 (reference) |
|
1.00 (reference) |
|
|
Young (<50) |
2.03 (1.21-3.40) |
0.007 |
1.55 (0.93-2.57) |
0.091 |
Table 4
Logistic regression analysis of independent contributions of variables for small (<5 mm) ruptured aneurysms in male patients over 50 years
|
OR (95% CI) |
p-value |
|
Age |
|
50-59 |
1.00 (reference) |
|
|
60-69 |
1.22 (0.6-2.47) |
0.581 |
|
70-79 |
1.13 (0.35-3.68) |
0.842 |
|
≥80 |
3.09 (0.52-18.55) |
0.217 |
|
|
Location |
|
ACoA |
0.45 (0.1-1.94) |
0.285 |
|
PCoA |
1.32 (0.25-6.98) |
0.746 |
|
ICA |
1.00 (reference) |
|
|
MCA |
0.55 (0.12-2.48) |
0.435 |
|
ACA |
1.11 (0.13-9.75) |
0.925 |
|
PC |
1.72 (0.17-17.48) |
0.646 |
|
|
Multiplicity |
|
No |
1.00 (reference) |
|
|
Yes |
0.44 (0.17-1.16) |
0.097 |
|
|
HTN |
|
No |
1.00 (reference) |
|
|
Yes |
0.84 (0.41-1.72) |
0.637 |
|
|
DM |
|
No |
1.00 (reference) |
|
|
Yes |
2.23 (0.65-7.67) |
0.204 |
|
|
Alcohol |
|
No |
1.00 (reference) |
|
|
Yes |
2.23 (1.03-4.84) |
0.042 |
|
|
Smoking |
|
No |
1.00 (reference) |
|
|
Yes |
0.66 (0.31-1.4) |
0.285 |
References
1. Beck J, Rohde S, Berkefeld J, Seifert V, Raabe A : Size and location of ruptured and unruptured intracranial aneurysms measured by 3-dimensional rotational angiography. Surg Neurol 65 : 18-25; discussion 25-27, 2006   2. Cebral JR, Raschi M : Suggested connections between risk factors of intracranial aneurysms: a review. Ann Biomed Eng 41 : 1366-1383, 2013   3. Dolati P, Pittman D, Morrish WF, Wong J, Sutherland GR : The frequency of subarachnoid hemorrhage from very small cerebral aneurysms (< 5 mm): a population-based study. Cureus 7 : e279, 2015    4. Fogelholm R, Murros K : Cigarette smoking and subarachnoid haemorrhage: a population-based case-control study. J Neurol Neurosurg Psychiatry 50 : 78-80, 1987    5. Hop JW, Rinkel GJ, Algra A, van Gijn J : Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 28 : 660-664, 1997   6. Inagawa T : Incidence and risk factors for multiple intracranial saccular aneurysms in patients with subarachnoid hemorrhage in Izumo city, Japan. Acta Neurochir (Wien) 151 : 1623-1630, 2009   7. International Study of Unruptured Intracranial Aneurysms InvestigatorsUnruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. N Engl J Med 339 : 1725-1733, 1998   8. Ishibashi T, Murayama Y, Saguchi T, Ebara M, Arakawa H, Irie K, et al : Justification of unruptured intracranial aneurysm repair: a single-center experience. AJNR Am J Neuroradiol 34 : 1600-1605, 2013    9. Jeong HW, Seo JH, Kim ST, Jung CK, Suh SI : Clinical practice guideline for the management of intracranial aneurysms. Neurointervention 9 : 63-71, 2014    10. Joo SW, Lee SI, Noh SJ, Jeong YG, Kim MS, Jeong YT : What is the significance of a large number of ruptured aneurysms smaller than 7 mm in diameter? J Korean Neurosurg Soc 45 : 85-89, 2009    11. Juvela S, Hillbom M, Numminen H, Koskinen P : Cigarette smoking and alcohol consumption as risk factors for aneurysmal subarachnoid hemorrhage. Stroke 24 : 639-646, 1993   12. Juvela S, Poussa K, Porras M : Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke 32 : 485-491, 2001   13. Kataoka K, Taneda M, Asai T, Yamada Y : Difference in nature of ruptured and unruptured cerebral aneurysms. Lancet 355 : 203, 2000  15. Komotar RJ, Mocco J, Solomon RA : Guidelines for the surgical treatment of unruptured intracranial aneurysms: the first annual J. Lawrence pool memorial research symposium--controversies in the management of cerebral aneurysms. Neurosurgery 62 : 183-193; discussion 193-194, 2008    16. Lee GJ, Eom KS, Lee C, Kim DW, Kang SD : Rupture of very small intracranial aneurysms: incidence and clinical characteristics. J Cerebrovasc Endovasc Neurosurg 17 : 217-222, 2015    17. Longstreth WT Jr, Nelson LM, Koepsell TD, van Belle G : Cigarette smoking, alcohol use, and subarachnoid hemorrhage. Stroke 23 : 1242-1249, 1992   18. Ohashi Y, Horikoshi T, Sugita M, Yagishita T, Nukui H : Size of cerebral aneurysms and related factors in patients with subarachnoid hemorrhage. Surg Neurol 61 : 239-245; discussion 245-247, 2004   19. Orz Y, Kobayashi S, Osawa M, Tanaka Y : Aneurysm size: a prognostic factor for rupture. Br J Neurosurg 11 : 144-149, 1997   20. Qureshi AI, Suarez JI, Parekh PD, Sung G, Geocadin R, Bhardwaj A, et al : Risk factors for multiple intracranial aneurysms. Neurosurgery 43 : 22-26; discussion 26-27, 1998   21. Qureshi AI, Sung GY, Suri MF, Straw RN, Guterman LR, Hopkins LN : Factors associated with aneurysm size in patients with subarachnoid hemorrhage: effect of smoking and aneurysm location. Neurosurgery 46 : 44-50, 2000   22. Rahman M, Ogilvy CS, Zipfel GJ, Derdeyn CP, Siddiqui AH, Bulsara KR, et al : Unruptured cerebral aneurysms do not shrink when they rupture: multicenter collaborative aneurysm study group. Neurosurgery 68 : 155-160; discussion 160-161, 2011    23. Seibert B, Tummala RP, Chow R, Faridar A, Mousavi SA, Divani AA : Intracranial aneurysms: review of current treatment options and outcomes. Front Neurol 2 : 45, 2011    24. Silva ÂR, Câmara RL, Valença MM : Carotid siphon geometry and variants of the circle of Willis in the origin of carotid aneurysms. Arq Neuropsiquiatr 70 : 917-921, 2012    25. Sonobe M, Yamazaki T, Yonekura M, Kikuchi H : Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke 41 : 1969-1977, 2010   27. Turan N, Heider RA, Zaharieva D, Ahmad FU, Barrow DL, Pradilla G : Sex differences in the formation of intracranial aneurysms and incidence and outcome of subarachnoid hemorrhage: review of experimental and human studies. Transl Stroke Res 7 : 12-19, 2016    28. Uchiyama S : Japanese guidelines for the management of stroke 2009. Nihon Ronen Igakkai Zasshi 48 : 633-636, 2011   29. van Gijn J, Kerr RS, Rinkel GJ : Subarachnoid haemorrhage. Lancet 369 : 306-318, 2007   30. Weir B, Disney L, Karrison T : Sizes of ruptured and unruptured aneurysms in relation to their sites and the ages of patients. J Neurosurg 96 : 64-70, 2002   31. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al : Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362 : 103-110, 2003   32. Wiebers DO, Whisnant JP, Sundt TM Jr, O’Fallon WM : The significance of unruptured intracranial saccular aneurysms. J Neurosurg 66 : 23-29, 1987   33. Yonekura M : Importance of prospective studies for deciding on a therapeutic guideline for unruptured cerebral aneurysm. Acta Neurochir Suppl 82 : 21-25, 2002  
|
|

















