INTRODUCTION
With the advent of aging society and development of radiological techniques, osteoporotic vertebral compression fracture (OVCF) has become more commonly diagnosed. Among which an increasing number of patients show progressive kyphosis or delayed neurologic compromise without responding to conservative treatments or minimal invasive treatments, including vertebroplasty or kyphoplasty. It is known that the incidence of nonunion is approximately 13.5% and that of intraverteral cleft (IVC) sign is about 7-13% in patients with OVCF18,33).
Also known as KummellŌĆÖs disease (KD) was defined by avascular necrosis of a vertebral body occurring in a delayed fashion after minor trauma, this disease requires a more aggressive treatment as opposed to OVCF, because when the disease is diagnosed, vertebral kyphosis and intravertebral instability have already been present with a high degree of vertebral collapse6,18,22,27,31). In 1895, Kummell et al. reported delayed posttraumatic vertebral collapse after asymptomatic minimal spine trauma, which was referred to as KD, and later KD was called by various names such as intravertebral pseudoarthrosis, delayed posttraumatic vertebral osteonecrosis, avascular necrosis of vertebral body, fracture nonunion, IVC, ischemic vertebral collapse, etc.18). As indicated by these confusing names, there is no known guideline for the accurate pathogenesis, radiological diagnosis methods and the best treatments for this disease until now3,7,18,25,37).
By referring to various articles, this paper aims to present recently recognized pathogeneses for this disease and help reduce its morbidity by performing reasonable treatments before the development of spinal deformity or instability from KD through early radiological diagnosis.
CLINICAL FEATURES
The most important clinical feature of KD is delayed vertebral collapse occurring after the history of minimal trauma5,29). In most cases, however, the early examination of trauma is not performed due to the lack of symptoms, and vertebral collapse is only confirmed with X-ray performed after symptoms appear22,35). When symptoms become gradually serious after the occurrence of asymptomatic trauma, conservative treatment is not that effective, and afterwards, delayed vertebral collapse or intravertebral vacuum cleft occurs6,22,29,35). The intervals from the occurrence of asymptomatic trauma, the onset of symptoms, and vertebral collapse vary widely35). The most common clinical stages of KD are the three stages of Steel and the five stages of Benedek, although many authors have reported the stages in a varied manner. Among these stages, it is common that there appears an asymptomatic period after initial minor trauma, and then symptoms become gradually serious, and finally intractable pain, vertebral collapse or neurologic compromise occurs in the last phase5,29). Neurological deficits rarely appear in the beginning, and vertebral collapse or vertebral pseudoarthrosis occurs only when symptoms become worse, in most cases5,21,28,35).
It is considered that KD is more commonly observed in women, given that our clinical experience suggests that OVCF occurs significantly higher in women, although some studies reported that OVCF takes place slightly higher in men among middle-aged adults35). The most common site of fracture is the thoracolumbar junction, which is similar to that of OVCF23,27,35). A certain authors argued that KD and OVCF are two different diseases based on the ground that both have different injury mechanisms (hyperflexion and lifting, respectively) and show different signals from magnetic resonance image (MRI), and that OVCF rarely presents neurological deficits and is common in men23,35). However, this study suggests that KD is a type of complication as well as the end stage of OVCF, because fractures easily occur due to hyperflexion in OVCF as well. The difference in signals from MRI between the two diseases is due to the discrepancy in the time of fracture, let alone many common aspects between the two diseases.
PATHOGENESIS
The representative hypothesis for the pathogenesis of KD is delayed healing or nonhealing by osteonecrosis of the fractured vertebrae16,21). The pathogenesis for osteonecrosis can be largely divided into two. First, it is caused by anatomical or mechanical damage to the blood vessels supplying the blood to the vertebral body. Normally, the blood supply to the thoracolumbar spine is through the paired segmental arteries, including anterior and posterior central arteries16). The former supplies the blood to the anterior 1/3 of the vertebral body, while the latter provides the blood to the lamina and posterior vertebral body. Osteonecrosis easily occurs in the anterior 1/3 of the vertebral body as the site is vulnerable to ischemic injuries because the anterior central artery is short with less collateral vessels formed16). Kim et al.16) estimated that when the midportion of the vertebral body is fractured, the stretching injury of the segmental artery occurs as the cross-sectional area of the fractured spine widens, resulting in vascular insult. This result was not statistically significant but was thought to be due to the small number of subjects in the study. Sugita et al.30) also reported that the fracture of a midportion type leads to vascular injury as opposed to that of an endplate type. Second, osteonecrosis can occur due to the pathology of the blood vessels supplying the blood to the vertebral body. Table 1 shows the risk factors and possible pathogenesis of osteonecrosis23). Chronic alcoholism, chronic steroid therapy and hemoglobinopathy become the risk factors that develop such pathology, and such risk factors cause fat accumulation of the medullary arteriole and develop artherosclerosis, aggravating the blood supply to the vertebral body16). In particular, the fat microembolism that occurs during the fracture blocks the medullary arteriole, worsening the blood supply to the vertebral body. These hypotheses delay neovascularization of the fractured vertebral body, and eventually resulting in nonunion or delayed healing of osteonecrosis. It was also reported that the accompanying poor blood supply, SchmorlŌĆÖs node and normal stress prevent bone healing5). There is a report that patients with serious osteoporosis show delayed healing due to the lack of absolute number in osteoblast, and that IVC sign occurs more often when bone mineral density is low9).
However, some authors argued that osteonecrosis can be seen in histopathological examination of the unfractured vertebral body, and it is very rare that it is accompanied by femoral head avascular necrosis2). They emphasized that biomechanical properties are important because intravertebral clefts occur more frequently in thoraco-lumbar junctions and compressed vertebral body itself is related to intravertebral instability14).
We suggest that the fractured vertebral body worsens the watershed zone blood supply of the vertebral body and especially the axial loading at the thoracolumbar junction increases the intravertebral instability due to the progression of the vertebral kyphosis. If the clinical risk factor is accompanied, osteonecrosis can be accelerated. However, it is not known yet which major risk factor accelerates osteonecrosis.
RADIOLOGIC FEATURES
The most important radiological finding of KD is IVC sign. Since an IVC sign is the poor prognostic factor for delayed vertebral collapse, many radiologic examinations have been conducted for the early detection of this factor. Maldague, who first mentioned IVC, suggested that such phenomenon is not commonly observed in neoplasm or infection, while other researchers reported that it can be seen in infection or tumor as well19,21,28,31). However, it was reported that malignancy and infection can be detected with the difference in signals from MRI34). There are also reports that IVC is sometimes observed in OVCF as well, but it is not seen in acute period19,25). The association between IVC sign and KD in osteonecrosis is not clearly known despite a number of previous studies, but IVC sign is an eventual finding that strongly suggests the presence of osteonecrosis, though it is not the pathognomic sign of KD21). Libicher et al.19) claimed that IVC was a specific sign of osteonecrosis in vertebral compression fractures, with 85% sensitivity, 99% specificity, and 91% positive predictive value. There is a theory that the IVC sign from osteonecrosis is caused when it is filled with vacuum gas or when intradiscal vacuum gas enters the intravertebral space. It was suggested that if the content is gas it is mostly nitrogen, while if it is fluid, it is a similar substance with synovial fluid, and that gas and fluid can change depending on body posture31). The finding of each radiographic risk factors are summarized in Table 2.
Simple radiography
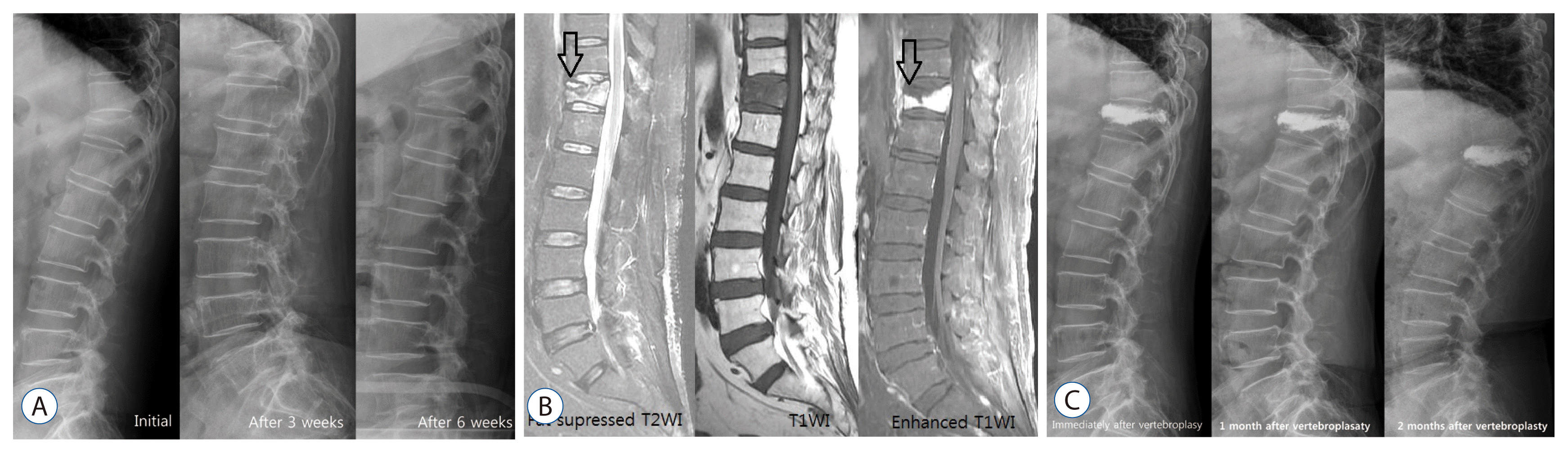
Early diagnosis of KD in simple radiography is difficult. However, serial follow images are very useful diagnostic tool (Fig. 1). Serial simple radiography could show deformd vertebral body, progressive compression fracture and kyphosis.
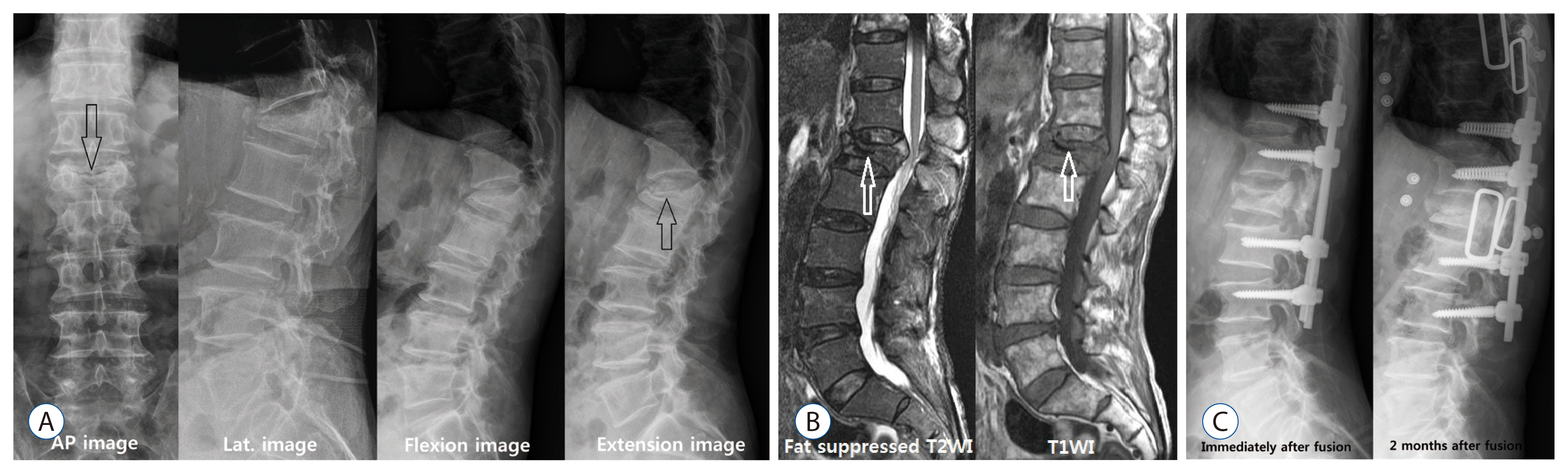
Another feature of KD shown in simple radiography is IVC sign. The IVC sign appears as a transverse, linear or semi-lunar radiopaque shadow of collapsed vertebrae and is more obvious in anteroposterior view, and it is most useful to check IVC sign by inducing intravertebral instability31). Since the presence of IVC sign implies intravertebral instability, the IVC sign could be well observed from images such as flexion-extension dynamic simple radiography which induces intravertebral instability (Fig. 2)8,24). The standing flexion and extension lateral radiographs, which are most commonly performed, can worsen vertebral fracture because the symptoms can be aggravated in flexion and thus it is hard to get patientsŌĆÖ cooperation. McKiernan et al.24) suggested the usefulness of supine cross-table lateral radiographs and recommended examination for evaluating dynamic mobility. Cho et al.8) suggested the cross lateral radiography was useful in identifying IVC, predicting the degree of restoration of postoperative vertebral heights and wedge angles, and provoked less pain during.
Computerized tomography (CT)
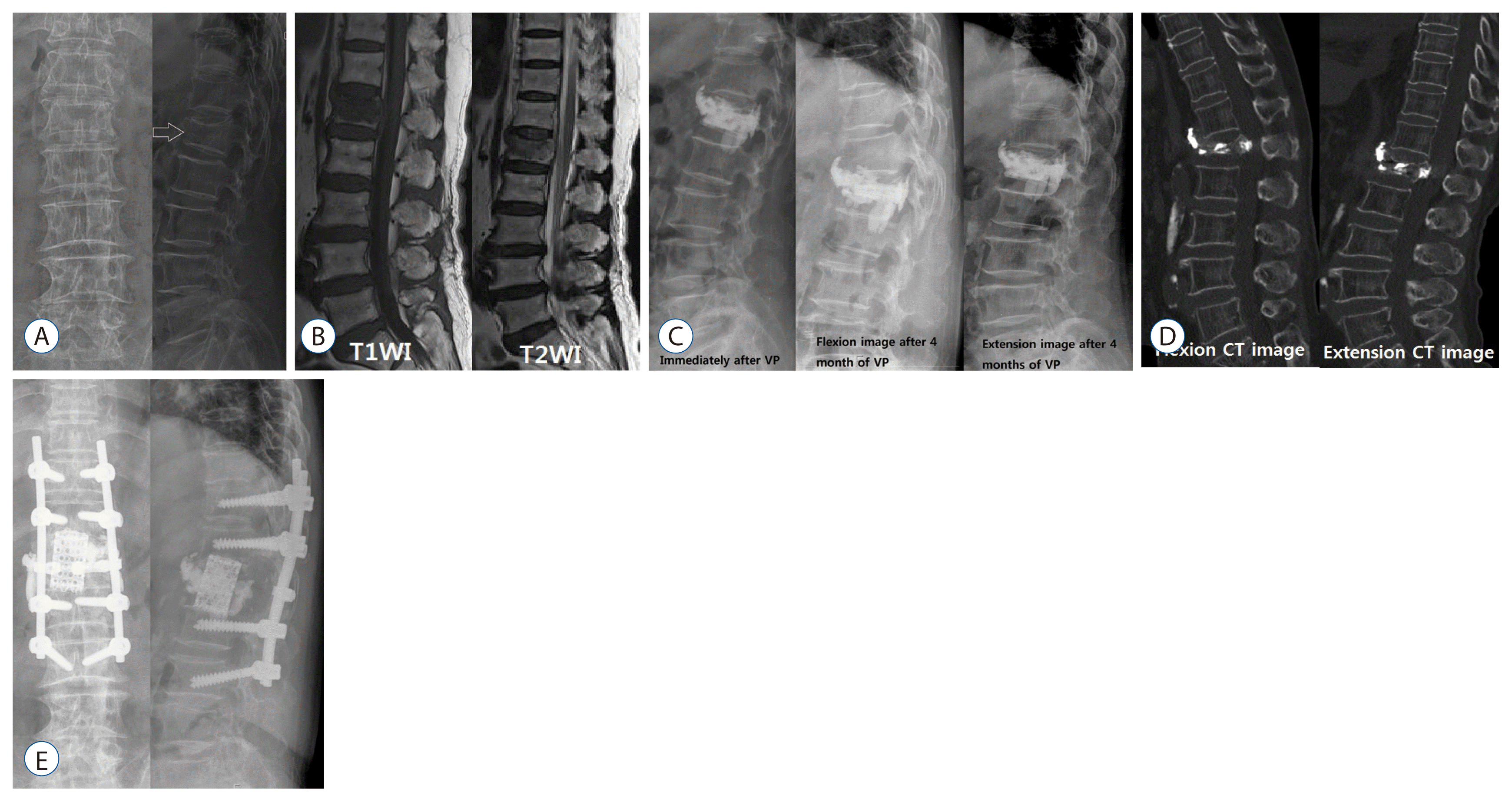
CT can show a clearer finding of bone fracture than X-ray, identify a heterogeneous and irregular shape of bone fragment, and accurately display the involved area of fracture19,31). In our experience, dynamic flexion-extension CT is a very useful tool for diagnosing IVC and spinal instability (Fig. 3).
MRI
MRI is the most useful diagnostic tool for the diagnosis of vertebral compression fracture and IVC, especially T1 weighted image and fat-suppressed T2 weight image1,4,13,19,36). The affected area shows low SI on T1WI and high SI on T2WI in the acute period, and then changes into iso-signal intensity as the bone marrow edema gradually decreases1). However, the signals of MRI differ depending on the diagnosis time, and the reported difference in MRI signals significantly vary by authors. Young et al.35) reported that osteonecrotic vertebral collapse shows increased signal intensity in T1 weight image, while decreased signal intensity in T2 weight image. However, most of studies revealed that osteonecrotic vertebral collapse shows decreased signal intensity in T1 weight image, while in T2 weight image, it displays variable signals, such as high in fluid and low in gas, depending on the contents of IVC1,4,13,19,31,36). It was reported that a ŌĆśdouble line signŌĆÖ, in which a high signal zone is shown in the center and a low signal zone in the periphery in T2WI at the fractured vertebral body, is associated with IVC (Fig. 1)36). There is also a report that this sign is very similar with the ŌĆścrescent sign,ŌĆÖ which is avascular necrosis of the femor16). While the early IVC detectability of noncontrast MRI is approximately 60.9%, that of contrast enhancing MRI increases up to 91.3% as the low SI area of necrotic content and enhancing vertebral body can be clearly distinguished, making an early diagnosis possible (Fig. 1)13).
In addition to IVC, there is another predictive factor in MRI for delayed vertebral collapse. Ahn et al.1) reported that early bone marrow pattern observed on initial MRI was a poor prognostic factor of OVCF if low SI on T1WI. Tsujio et al.32) reported that thoracolumbar junction, middle-column injury, and confined or diffuse low SI on T2WI are the predictive factors of nonunion, and in particular that confined low SI on T2WI means repetitive mechanical compression due to a tensile and shearing force, and thus the confined type can be more nonunion than diffuse low SI on T2WI. Ha and Kim11) reported that the possibility of progressive vertebral collapse is higher in midportion type fracture and posterior wall combined fracture, as well as when kyphotic angle >10 degrees and height loss >15% of the initial value, and when an IVC sign is present. Sugita also divided osteoporotic spinal fracture by radiologic features and reported that delayed vertebral collapse and IVC can be present in swelled-front type and dented type fractures30).
Radionuclide bone image
Radionuclide bone imaging is not specific to diagnosing KD, but it is a good modality for screening for many pathologic conditions20). When vascular insufficiency occurs in the bone, osteonecrosis occurs within 12 hours. In the acute phase (<12 hr), a radiopacic effect occurs in the nuchal part of the affected bone, and after the revascularization, there is an intense radiotracer uptake due to active osteoblastic repair20). After bone remodeling, the radiotracer uptake decreases to baseline20). Radionuclide bone images appear to be hot uptake in many KD cases, but rarely have been reported as cold uptake15). Matzaroglou et al.22) reported that a single photon emission tomography/computed tomography was performed in KD patients and that the degree of uptake could determine the degree of chronicity.
TREATMENT
The most important treatment method is a conservative treatment. However, a more aggressive treatment is needed when there is intractable pain, a degree of kyphotic deformity or neurologic compromise7,17,18,25-27,34,37,38). In our institution, if the patient is diagnosed with KD before the vertebral collapse, first of all, conservative treatment with an osteoanabolic agent should be performed. Considering vertebroplasty (VP) or kyphoplasty (KP) and short segment instrumental screw fixation in patients without severe kyphosis or neurologic compromise and with uncontrollable pain, vertebral replacement is considered in patients with severe kyphosis and neurologic compromise
Conservative treatment
The most important treatment of OVCF without the presence of neurological deficits is a conservative treatment represented by bed rest, nonsteroidal anti-inflammatory drugs medication and orthosis7,17,18,25-27,34,37,38). As for medication, biphosphonate, Calcium formula and vitamin D are used, the same as in the treatment of osteoporosis. However, the risk factors of progressive delayed vertebral collapse from MRI do not respond well to conservative treatments in many cases, and thus require a more aggressive treatment. In conservative treatment, about 60% of OVCF patients have improved pain for about 3 months33). In the remaining patients, pain is not controlled or controlled by analgesics33). Although the predictors of chronic pain were unknown, other authors reported that early ambulation and prolonged bed rest also affected bone nonunion18,33). A recent study showed that the osteoanabolic agent, known to be used for the treatment of OVCF, is effective for patients with IVC sign and instability10).
Minimal invasive treatment
VP and KP can be considered when pain remains or progressive vertebral collapse occurs even after conservative treatments are performed. VP and KP for OVCF are all effective, while KP is effective for reduction of vertebral body height, but there is no significant difference in pain control between VP and KP3,5). Some reported that when an IVC sign is present, polymethyl methacrylate (PMMA) is not an appropriate scaffolding for the fractured vertebral body as PMMA cannot be interdigitated with the trabeculae of the vertebral body, because the fibrocartilaginous membrane is formed inside the IVC, resulting in vertebral compression fracture, which makes intravertebral PMMA be dislodged to the spinal canal, possibly causing neurologic compromise18). However, another author reported that the leakage of PMMA can be reduced due to the fibrocartilaginous membrane during VP17). Since the presence of IVC indicates intravertebral pseudoarthrosis, it can be seen as a good sign to restore collapsed vertebral height. However, it can also be a bad sign because many authors reported that an IVC sign can imply the leakage of PMMA in the spinal canal. It was reported that PMMA leakage is determined by the presence of IVC sign, viscosity of injected cement, injection volume and velocity, and the morphology of the fractured vertebral body26).
Heo et al.12) reported that vertebral recollapse occurs at the rate of approximately 3.2% after VP, suggesting that recollapse takes place when osteonecrosis occurs, or when osteonecrosis is aggravated as the implanted PMMA is a solid lump type. The solid type of implanted PMMA does not interlock with surround normal trabebulae because it does not have biocompatibility and remains as a simple space occupying material18). It develops recollapse, resulting in the aggravation of kyphotic angulation, creating a vicious cycle18). Therefore, if there is osteonecrosis or pseudoarthrosis in the fractured vertebra, VP and KP can be a contraindication12).
It is known that the difference in the effectiveness of VP and KP is not significant, and the correction of kyphotic angle is better in KP than VP, without much significance3,5). It was suggested that it is important to fully fill the cavity using sufficient PMMA by increasing the size of IVC and restoring vertebral body height with the inducement of hyperlordosis during surgery, and it is meaningful to use a cavitogram to check the process13). It was also shown that the distribution of homonymous PMMA can be obtained through VP by inserting the needle in the accurate IVC location with a contrast enhanced MRI13,25). However, it was reported that the larger the restored vertebral height is, the more recollapse occurs (Figs. 1 and 2)11).
Surigcal treatment
Surgical treatments are provided when intractable pain, neurologic deficit or progressive kyphosis is present7,18,34,37,38). The causes of delayed neurologic deficits include progressive kyphosis, retropulsed bony fragment and intravertebral instability, among which the most important cause is intravertebral instability (Figs. 2 and 3)7,18,34,37,38). The surgical outcomes of anterior approach and posterior approach are similar. However, the correction of kyphosis is better in anterior approach than in posterior approach, but its operating time is longer, and has a larger damage to the internal organ and blood loss34). Restoration of the fractured vertebral height is the most important, and the degree of correction of kyphosis is not related to the degree of pain relief7). However, there are often limitations in deciding the scope and time of surgery because most of patients undergoing this surgery are older, have osteoporosis, and are accompanied by other internal diseases7,18,34,37,38). Although many cases require vertebral body replacement and long level screw fixation, modified surgeries, such as VP or KP with short segmental screw fixation have been recently reported7,18,34,37). This modified surgery is considered to be a suitable method for elderly patients with various diseases and it is reported that the surgical results are very good7,18,34,37). The findings of osteonecrosis or intravertebral pseudoarthrosis from radiologic imaging indicate a higher possibility of delayed vertebral collapse, which is thought to require surgical treatment than conservative treatment or minimal invasive treatment. Like Fig. 3, when progressive vertebral collapse occurs after injecting PMMA in patients with osteonecrosis, vertebral replacement and long segmental screw fixation are required, and thus a more aggressive treatment is needed in the early stage when risk factors are present.
CONCLUSION
Delayed verterbral collapse after minimal asymptomatic trauma is not a very rare condition, and so far it is thought to be caused by a vascular insult. This disease is considered to be a complication of osteoporotic vertebral compression fracture. If there is a progression to osteonecrosis in the radiological examination at the initial stage of fracture, more active treatment is needed than the treatment of general compression fracture