Kim, Hyun, Kim, and Jahng: Surgical Outcomes According to Dekyphosis in Patients with Ossification of the Posterior Longitudinal Ligament in the Thoracic Spine
Abstract
Objective
Ossification of posterior longitudinal ligament (OPLL) in the thoracic spine may cause chronic compressive myelopathy that is usually progressive, and unfavorable by conservative treatment. Although surgical intervention is often needed, the standard surgical method has not been established. Recently, it has been reported that posterior decompression with dekyphosis is effective surgical technique for favorable clinical outcome. The purpose of this study was to evaluate the surgical outcomes in patients with thoracic OPLL according to dekyphosis procedure and to identify predictive factors for the surgical results.
Methods
A total of 25 patients with thoracic OPLL who underwent surgery for myelopathy from May 2004 to March 2017, were retrospectively reviewed. Patients with cervical myelopathy were excluded. We assessed the clinical outcomes according to various surgical approaches. The modified Japanese orthopedic association (JOA) scores for the thoracic spine (total, 11 points) and JOA recovery rates were used for investigating surgical outcomes.
Results
Of the 25 patients, 10 patients were male and the others were female. The mean JOA score was 6.7┬▒2.3 points preoperatively and 8.8┬▒1.8 points postoperatively, yielding a mean recovery rate of 53.8┬▒31.0%. The mean patientsŌĆÖ age at surgery was 52.4 years and mean follow-up period was 40.2 months. According to surgical approaches, seven patients underwent anterior approaches, 13 patients underwent posterior approaches, five patients underwent combined approaches. There was no significant difference of the surgical outcomes related with different surgical approaches. Age (Ōēź55 years) and high signal intensity on preoperative magnetic resonance (MR) image in the thoracic spine were significant predictors of the lower recovery rate after surgery (p<0.05). Posterior decompression with dekyphosis procedure was related to the excellent surgical outcomes (p=0.047). Dekyphosis did not affect the complication rates.
Conclusion
In this study, our result elucidated that old age (Ōēź55 years) and presence of intramedullary high signal intensity on preoperative MR images were risk factors related to poor surgical outcomes. In the meanwhile, posterior decompression with dekyphosis affected favorable clinical outcome. Posterior approach with dekyphosis procedure can be a recommendable surgical option for favorable results.
Key Words: Ossification of posterior longitudinal ligament ┬Ę Thoracic vertebrae ┬Ę Kyphosis ┬Ę Treatment outcome.
INTRODUCTION
Ossification of posterior longitudinal ligament (OPLL) in the thoracic spine is rare condition compared with cervical OPLL depending on the previous reports [ 4, 17, 20, 21, 23]. For this reason, characteristics of thoracic OPLL have not been adequately addressed. Thoracic OPLL often produces chronic compressive myelopathy that is usually progressive and unfavorable by conservative treatment. Therefore, surgical intervention is usually needed, surgical technique and decision making are also important. This is because of the high risk of complication related with surgery [ 19]. Different results of various surgical approaches and techniques have been reported [ 2, 8, 13- 15, 25]. Posterior approach is considered to be relatively safe, although definitive results of an optimal surgical method have not been presented. Recently, it has been reported that posterior approach with kyphosis correction is effective surgical method for decompression through posterior migration of the spinal cord [ 27]. We performed the surgery mainly through the posterior approaches, and we focused on the effect of dekyphosis procedure for treating thoracic OPLL. The purpose of this study was to evaluate the surgical outcomes in patients with thoracic OPLL according to dekyphosis procedure and to investigate the predictive factors for the surgical outcomes.
MATERIALS AND METHODS
This report was approved by Institutional Review Board (IRB No. : B-1911-577-109). Between May 2004 and March 2017, 25 patients, who underwent surgery for thoracic myelopathy due to OPLL, included in this study. The patients with cervical myelopathy were excluded. They were observed for a minimum of 1 year postoperatively. We evaluated the severity of patientsŌĆÖ myelopathy before and after surgery using the Japanese Orthopedic Association (JOA) scores for the thoracic spine (total, 11 points), which was derived from the JOA scoring system for cervical myelopathy by eliminating the motor and sensory scores for the upper extremities ( Table 1). In addition, surgical outcomes was evaluated with JOA recovery rate that were calculated by the following formula : (postoperative JOA score - preoperative JOA score) / (11 - preoperative JOA score) ├Ś 100%. The JOA scores were checked before surgery, a week, 3 months, 1 year after the surgery, and at the final follow-up visit. Recovery rates were calculated by comparing the final follow-up JOA score with the preoperative JOA score.
The mainly affected lesions were classified as upper (T1- T4), middle (T5-T8), or lower lesions (T9-T12) on the basis of the level of thoracic spine where they exert maximum compression on T2 weighted sagittal magnetic resonance (MR) image. We also noted presence of intramedullary high signal intensity on T2 weighted MR images in the thoracic spine, except for two unidentifiable images from outside. Ossification size was defined as an antero-posterior length of OPLL in the axial computed tomography (CT) image of the maximal compression level. Degree of cord compression was computed OPLL length divided by spinal diameter. Morphology of OPLL was classified into three types (flat, beak, or mixed) according to previous reports [ 1, 18]. Surgical treatment was performed by four attending neurosurgeons. They evaluated the clinical and radiological status of the patients and selected the surgical method based on their consent. There were cases in which kyphosis correction was attempted. At first, dekyphosis was performed through intraoperative positioning using Jackson spine table. In addition, further dekyphosis was achieved by surgical facetectomy/posterior column osteotomies and in site bending after rod installation ( Fig. 1). Dekyphosis was defined as the decrease of CobbŌĆÖs angle after surgery based on fused thoracic spinal segments. Intraoperative and postoperative complications were reviewed. Motor deterioration was defined as more one grade worsening of motor power rather than preoperative condition using the criteria of Medical research council scale.
Data were analyzed using the SPSS version 24 software (IBM Corp., New York, NY, USA). Statistical analysis was performed by studentŌĆÖs t-test and one way analysis of variance for comparing with surgical outcome according to patientsŌĆÖ factors and surgical approaches. FisherŌĆÖs exact test is used for evaluation of complication rates. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Demographics
The mean patientsŌĆÖ age at surgery was 52.4┬▒11.0 years and mean follow-up period was 40.2┬▒26.6 months. The mean JOA score was 6.7┬▒2.3 points preoperatively and 8.8┬▒1.8 points postoperatively, yielding a mean recovery rate of 53.8┬▒31.0%. Duration of symptoms before surgery was 18.7┬▒32.5 months, and nine patients (36%) had cervical OPLL history. Patients with old age (Ōēź55 years) were related to poor recovery rate ( p=0.015, Table 2).
Surgical outcomes related with radiological features
In the radiological exam, ossification of ligamentum flavum was detected in 14 patients (56%), intramedullary high signal intensity in preoperative MR image in 17 patients (68%), and lesions that limited single-level in 10 patients (40%). Patients with intramedullary high signal intensity on preoperative MR image showed significantly lower preoperative, postoperative JOA scores and recovery rate than patients without that ( p=0.048). Patients who limited single-level lesions had higher preoperative and postoperative JOA scores than patients with multiple-segment lesions, but difference of recovery rate was not significant between two groups. Patients with cord compression more than 50% and with ossification length more than 6 mm were identified as 14 patients equally. Beak type OPLL was the most frequent occurred group among the three categories according to morphology and did not affect surgical outcome. The preoperative JOA score of middle thoracic lesion was significantly low compared with upper or lower thoracic spine ( p=0.001, Table 2).
Surgical procedures
Surgical treatment was performed seven (28%) anterior approaches, 13 (52%) posterior approaches, and five (20%) combined approaches. Seventeen patients (68%) were enforced by instrumentation. There was no significant difference of the surgical outcomes related with different surgical approaches ( Table 3). However, the recovery rate tended to be low in the posterior decompression alone groups compared with instrumented group ( Table 4). Dekyphosis was acquired in seven patients. But, only four cases belonging to the posterior approach group were intended for kyphosis correction during operation. The recovery rate of these four patients was 83.3%┬▒23.6 and showed excellent surgical outcome compared to other patients ( p=0.047, Table 5). Among four cases, two patients recovered to normal and the other two recovered partially ( Table 6).
Complications
One or more complications were occurred in eight patients (32%), including intra-operative dural tear in five patients (20%), neurologic deterioration in four patients (16%), and epidural hematoma required reoperation in two patients (8%). Complication rates according to the approach were not statistically significant ( Table 7). Dekyphosis did not affect the complication rates ( Table 8). Dural tears were successfully sutured during surgery, and patients with motor weakness recovered after conservative treatment or epidural hematoma evacuation. There was no permanent problem related with complications. Complications did not have a significant effect on the surgical outcomes ( Table 9).
DISCUSSION
Thoracic OPLL has been reported in the range of 0.8% to 1.9% [ 1, 16]. In cases of neurological deterioration, surgical treatment is often required. According to the directions of approaches, surgical methods may be divided into anterior, posterior, combined approaches, and instrumentation may be considered depending on the patientsŌĆÖ neurologic status and radiologic findings. Circumferential decompression that is anterior decompression with posterior approach was suggested as one of the surgical procedure [ 3]. Methods of removing the beak-type OPLL using an ultrasonic osteotome also have been introduced [ 10]. In theoretically, OPLL is compressing in front of the spinal cord, so it is desirable to remove the cause of compression through an anterior approach. However, direct removal of OPLL has a high risk of complications such as dural tear and neurologic deficit, etc [ 12, 24, 26]. Diverse results of surgical techniques have been reported [ 2, 5, 8, 13- 15, 25]. Posterior approach is considered to be relatively safe, but the guidelines for surgical treatment have not yet been clarified. We have been performed various approaches. However, posterior approach and instrumentation have been in place in 10 patients (10/11, 90.9%) since January 2013, and have shown good surgical outcomes. Especially, posterior decompression with dekyphosis showed excellent outcome after surgery. Koda et al. [ 12] suggested that dekyphosis should not be performed after posterior decompression because kyphosis correction may cause instrument failure. Hyun et al. [ 6] also reported instrument failure following dekyphosis for treating adult spinal deformity. But, Zhang et al. [ 27] confirmed by MR image that posterior migration and complete decompression of the spinal cord was possible through posterior approach with kyphosis correction. We performed posterior decom pression with dekyphosis in four patients, and experienced full recovery in two patients (patients 1 and 2) and partial recovery in two patients (patients 3 and 4) ( Table 6). We thought that the preoperative JOA score was as high as 8 points, which might be a contributing factor that could complete recovery in two patients. The remaining two patients had a relatively low preoperative JOA score of 5 points, but one patient (patient 3) had a high recovery rate of 83.3%. One (patient 3) had a greater angle of kyphosis correction compared to the other patient (patient 4), and the spinal cord was effectively decompressed, resulting in a good clinical outcome ( Figs. 2 and 3). Thoracic spine has physiological kyphosis, and OPLL compress the spinal cord anteriorly. In the posterior approach, direct OPLL removal is difficult, but even more effective indirect decompression can be performed by dorsally shifting the spinal cord through dekyphosis without removing it directly. In this study, instrument failure did not occur. Therefore, posterior decompression with dekyphosis seemed to be a way to maximize the purpose of decompression by lowering the complication of the anterior approach. Several institutions have reported that only posterior decompression may worsen the cord compression by progressive thoracic kyphosis [ 1, 12, 16, 19]. In our experience, recovery rates tended to lower in only posterior decompression group. We could identify that the compression of the spinal cord became worse as shown Fig. 2 after laminectomy alone. There is a limit to generalize the low recovery rate of the posterior decom pression alone group from our study. Because only two patients included in this group, and the low preoperative JOA score might inhibit recovery. We reported that patients with single-level OPLL had a higher preoperative and postoperative JOA scores than the other patients. Hu et al. [ 3] reported 26 patients who underwent circumferential decompression, the recovery rate was lower as the level of OPLL was increased. We applied various surgical procedures for multilevel OPLL, and the surgical outcome of multilevel OPLL was not statistically lower than that of singelevel OPLL. The thoracic spinal cord is the watershed area of the spinal circulation, which may have affected neurologic status due to a decrease in blood supply in the presence of multilevel lesions. However, if appropriate surgery is performed according to the patientsŌĆÖ status, satisfactory results will be obtained as in this study. Clinically, old age (Ōēź55 years) was related to poor surgical outcome. Patients included in the old age group had a low recovery rate although the preoperative neurologic status was not bad compared to the young age group. Xu et al. [ 24] noticed that complication rates of surgical treatment for thoracic OPLL and patientsŌĆÖ age were independent different to cervical OPLL. We suggest that degradation of functional recovery capacity play a major role in poor clinical course. The data of the current study indicate that patientŌĆÖs age can substantially influence the surgical outcome of thoracic OPLL. It is necessary to inform the patients and their caregivers about the prognosis following surgery. Surgeons should agonize to increase the recovery capacity of elderly patients. In radiological exam, intramedullary signal change on preoperative MR image affected severe preoperative, postoperative neurologic deficits and poor surgical outcome. On the other hand, several factors (ossification length, Ōēź6 mm vs. <6 mm; degree of cord compression, Ōēź50% vs. <50%; morphologic type, flat vs. beak vs. mixed) did not affect surgical outcomes. Several articles have reported that the beak-type OPLL promotes a stressful situation in the spinal cord, leading to poor surgical outcome [ 7, 19]. However, in this study, the surgical outcome of patients with beak type OPLL was not poor. Many authors thought that intramedullary high signal intensity in MR image is evidence of pathologic damage to the spinal cord [ 11]. The pathology is considered range from acute edema to chronic myelomalacia [ 1, 9, 22]. In Thoracic OPLL, signal change of spinal cord is the result of accumulation of microtrauma due to persistent mechanical stress from ossified lesions. It reduces the reversible resilience of the spinal cord and results in poor surgical outcome. As a result, the shape or degree of compression of the OPLL is not critical, but the presence of intramedullary high signal intensity in MR image is considered to be the most important radiologic factor for surgical outcome. We did not find out a significant association between the occurrence of complications and the surgical outcome. Hu et al. [ 3] reported that cerebrospinal fluid leakage by dural tear with circumferential decompression of multilevel thoracic OPLL did not affect the surgical outcome. Xu et al. [ 24] noted that the complication rates tended to be negatively correlated with the postoperative JOA score. We used intraoperative neuromonitoring during every surgery. We irrigated operating field by warm saline and raised mean arterial pressure when intraoperative neuromonitoring was deteriorated. In the thoracic spine, OPLL itself may cause additional neurological impairment after laminectomy alone with increased compression of the spinal cord. Therefore, we subsequently performed laminectomy after installing a rod to prevent additional ventral compression of the spinal cord during surgery. We underwent suture in case of dural tear during surgical procedure and occasionally reinforced with fat tissue to minimize the cerebrospinal fluid leakage. MR images were obtained when postoperative motor paralysis occurred, hematoma evacuation was performed on two cases with epidural hematoma, and all of them recovered from neurological deteriorations. Complications are capable of overcoming to cope with the general management, and thus complication does not affect to have a significant effect on the poor surgical outcome. In other paper, they reported that if complications were treated properly, most complications can be recovered with satisfactory outcomes [ 26]. There are some limitations of present study, including it retrospective nature, small number of patients, and various surgical procedures, all of which may make it difficult to appeal statically significant conclusions. Nevertheless, this was a single institution study of patients with thoracic OPLL, a rare disease, who were treated surgically. We desire that the surgical results may afford basic data in support of the predictors for treating thoracic OPLL.
CONCLUSION
Posterior approach with dekyphosis is a relatively safe and effective procedure for the management of thoracic OPLL which can be a recommendable surgical option for favorable results. Our study showed that old age (Ōēź55 years) and presence of intramedullary high signal intensity in preoperative MR image in the thoracic spine were associated with poor surgical outcomes. Considering these factors, surgeons should be aware of the importance of planning in appropriate surgical treatments for thoracic OPLL.
Fig.┬Ā1.
Illustration of the surgical effect of posterior decompression with dekyphosis. A : Preoperative status. B : Posterior decompression with pedicle screw fixation. C : After dekyphosis procedure. 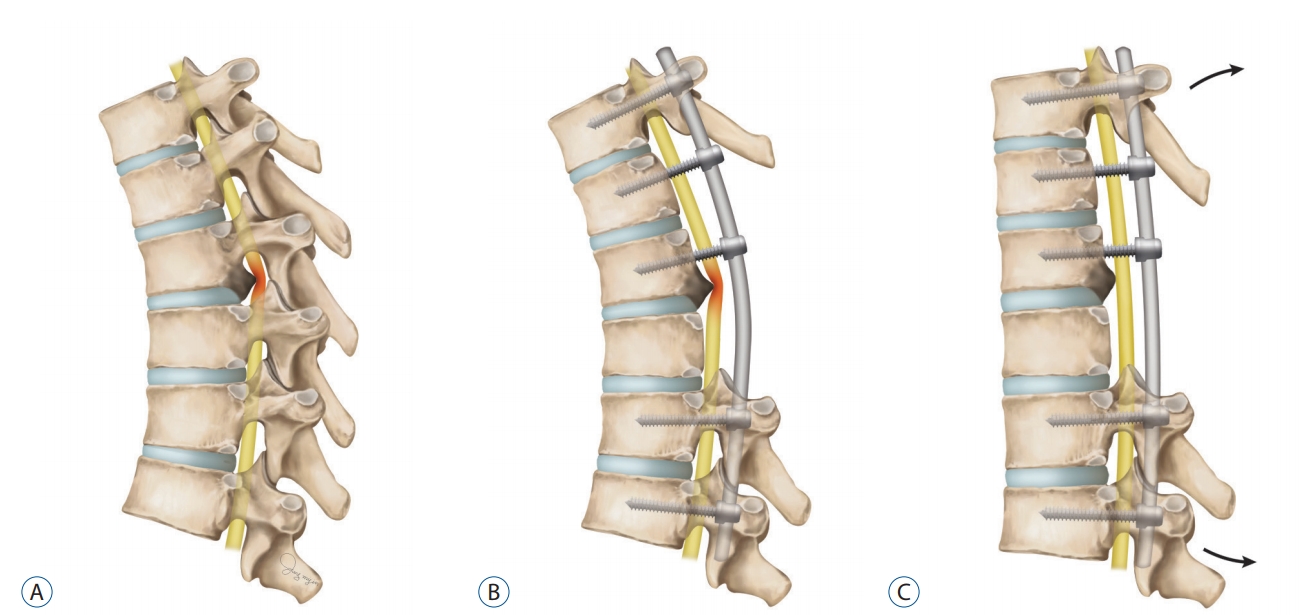
Fig.┬Ā2.
A 54-year-old female (patient No. 3) presented with lower extremities weakness. She underwent decompressive laminectomies without instrumentation at the T1-3 levels in another hospital and then increased the Cobb's angle from 9.3┬░ (A) to 9.5┬░ (B), resulting in progression of compressive myelopathy (D and E). We performed posterior instrumented fusion with dekyphosis from T1 to T4 levels (C). After revision surgery, we obtained an effective decompression of the spinal cord (F), and her neurological status was significantly improved (recovery rate=83.3%). 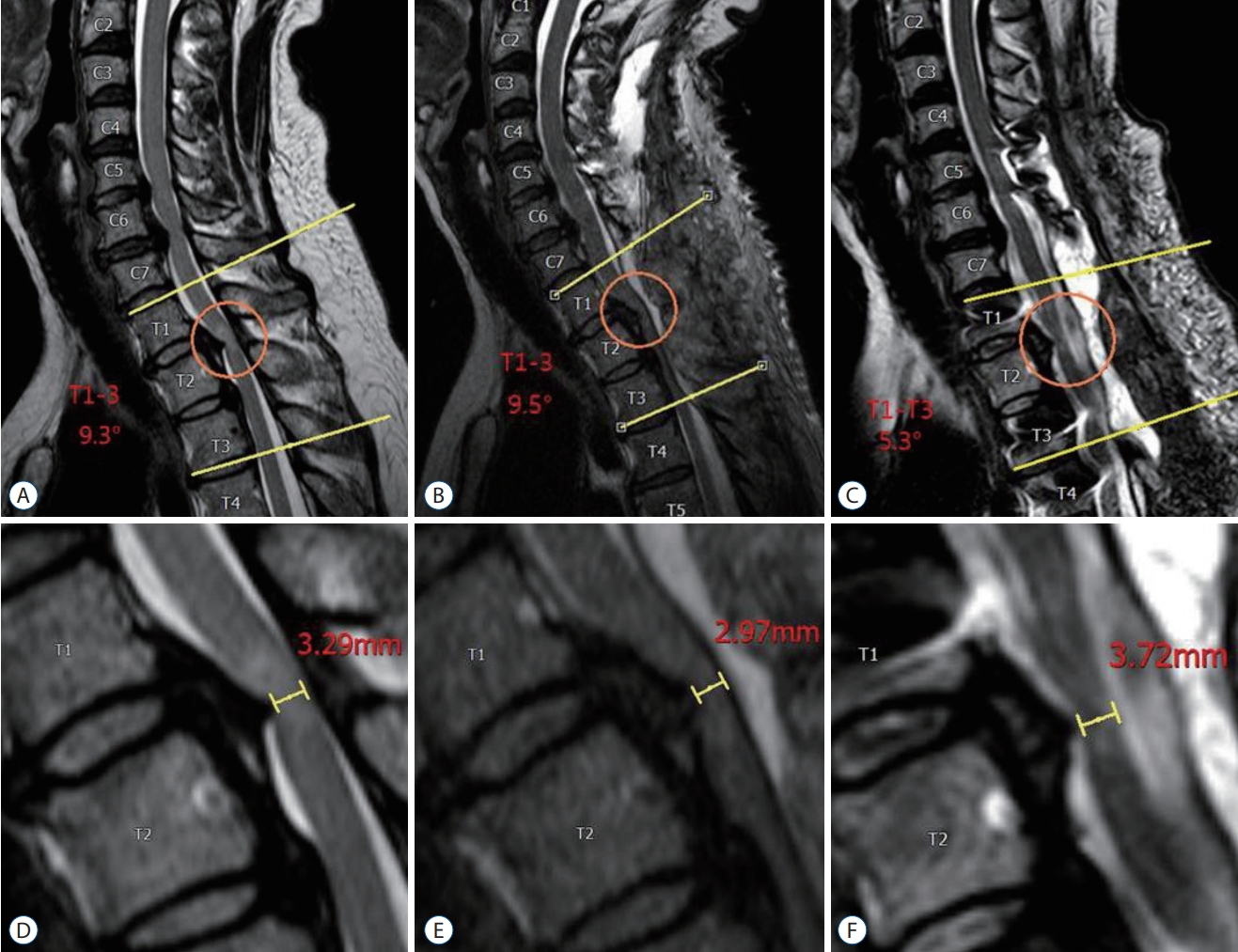
Fig.┬Ā3.
A 48-year-old female (patient No. 4) presented with lower extremities weakness. She underwent posterior decompression and instrumented fusion with dekyphosis from T1 to T7 levels (A and B). Postoperative anterior-posterior diameter of the spinal cord became widening (C and D), and her neurological status was substantially improved (recovery rate=50%). 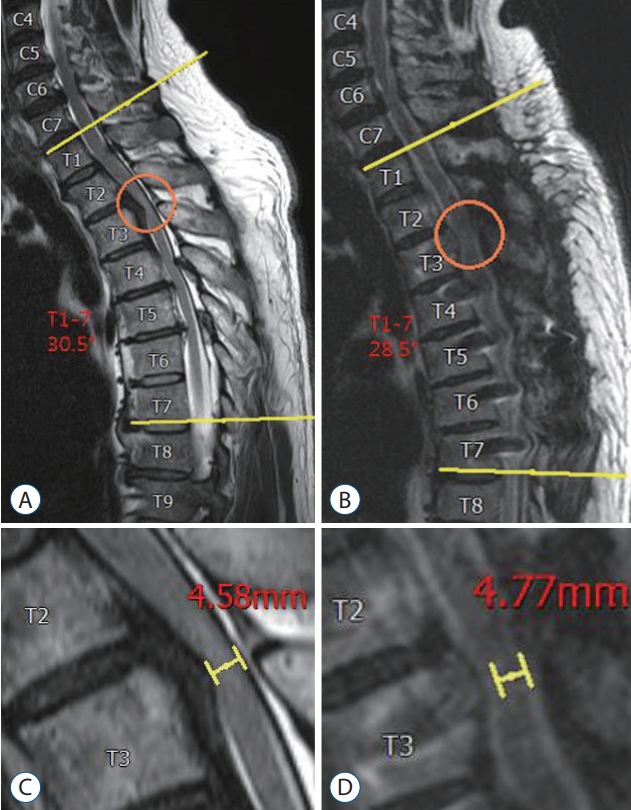
Table┬Ā1.
Summary of angiographic and clinical outcomes
|
Category |
Score (points) |
|
A. Lower extremity motor function |
|
|
ŌĆāUnable to stand up and walk by any means |
0 |
|
ŌĆāUnable to walk without a cane or other support on a level |
1 |
|
ŌĆāWalks independently on a level but needs support on stairs |
2 |
|
ŌĆāCapable of fast walking but clumsy |
3 |
|
ŌĆāNormal |
4 |
|
B. Sensory function |
|
|
ŌĆāI. Lower extremity |
|
|
ŌĆāŌĆāApparent sensory loss |
0 |
|
ŌĆāŌĆāMinimal sensory loss |
1 |
|
ŌĆāŌĆāNormal |
2 |
|
ŌĆāII. Trunk |
|
|
ŌĆāŌĆāApparent sensory loss |
0 |
|
ŌĆāŌĆāMinimal sensory loss |
1 |
|
ŌĆāŌĆāNormal |
2 |
|
C. Bladder function |
|
|
ŌĆāUrinary retention and/or incontinence |
0 |
|
ŌĆāSense of retention and/or dribbling and/or thin stream |
1 |
|
ŌĆāUrinary retardation and/or pollakisuria |
2 |
|
ŌĆāNormal |
3 |
|
Total score for normal patient |
11 |
Table┬Ā2.
Preoperative and postoperative neurological status related with patientsŌĆÖ factors
|
Value |
Pre Op. JOA score |
p-value |
Post Op. JOA score |
p-value |
Recovery rates |
p-value |
|
Sex |
|
|
0.374 |
|
0.552 |
|
0.559 |
|
ŌĆāMale |
10 |
7.2┬▒2.4 |
|
9.1┬▒1.5 |
|
49.7┬▒33.7 |
|
|
ŌĆāFemale |
15 |
6.3┬▒2.2 |
|
8.7┬▒2.1 |
|
57.7┬▒31.3 |
|
|
Age (years) |
|
|
0.475 |
|
0.259 |
|
0.015 |
|
ŌĆāŌēź55 |
10 |
7.1┬▒2.4 |
|
8.3┬▒2.0 |
|
35.2┬▒30.9 |
|
|
ŌĆā<55 |
15 |
6.4┬▒2.2 |
|
9.2┬▒1.7 |
|
67.3┬▒26.1 |
|
|
Symptom duration (months) |
|
|
0.458 |
|
0.647 |
|
0.339 |
|
ŌĆāŌēź6 |
11 |
7.1┬▒2.7 |
|
8.6┬▒2.2 |
|
47.1┬▒38.0 |
|
|
ŌĆā<6 |
14 |
6.4┬▒2.0 |
|
9.0┬▒1.6 |
|
60.3┬▒26.0 |
|
|
OPLL in the cervical spine |
|
|
0.484 |
|
0.325 |
|
0.583 |
|
ŌĆāYes |
9 |
7.1┬▒2.2 |
|
9.3┬▒1.8 |
|
59.7┬▒37.3 |
|
|
ŌĆāNo |
16 |
6.4┬▒2.4 |
|
8.5┬▒1.9 |
|
51.5┬▒29.2 |
|
|
OLF |
|
|
0.107 |
|
0.218 |
|
0.853 |
|
ŌĆāYes |
14 |
6.0┬▒1.9 |
|
8.4┬▒1.8 |
|
53.3┬▒28.2 |
|
|
ŌĆāNo |
11 |
7.5┬▒2.5 |
|
9.4┬▒1.9 |
|
55.9┬▒37.4 |
|
|
High signal intensity on MR image |
|
|
0.001 |
|
0.001 |
|
0.048 |
|
ŌĆāYes |
17 |
5.8┬▒2.1 |
|
8.1┬▒1.7 |
|
42.5┬▒19.8 |
|
|
ŌĆāNo |
6 |
9.0┬▒1.3 |
|
10.7┬▒0.8 |
|
83.3┬▒40.8 |
|
|
OPLL type |
|
|
0.090 |
|
0.246 |
|
0.752 |
|
ŌĆāFlat |
3 |
8.0┬▒2.6 |
|
9.3┬▒2.0 |
|
61.0┬▒34.8 |
|
|
ŌĆāBeak |
17 |
7.0┬▒2.3 |
|
9.1┬▒1.8 |
|
56.1┬▒34.5 |
|
|
ŌĆāMixed |
5 |
4.8┬▒1.1 |
|
7.6┬▒1.8 |
|
45.0┬▒24.0 |
|
|
Cord compression (%) |
|
|
0.183 |
|
0.395 |
|
0.963 |
|
ŌĆāŌēź50 |
14 |
6.2┬▒2.0 |
|
8.6┬▒1.9 |
|
55.1┬▒27.0 |
|
|
ŌĆā<50 |
11 |
7.5┬▒2.5 |
|
9.2┬▒1.8 |
|
55.7┬▒38.9 |
|
|
Affecting level |
|
|
0.033 |
|
0.042 |
|
0.666 |
|
ŌĆā1 |
10 |
7.8┬▒1.8 |
|
9.7┬▒1.4 |
|
58.3┬▒40.5 |
|
|
ŌĆāŌēź2 |
15 |
5.9┬▒2.3 |
|
8.3┬▒1.9 |
|
51.9┬▒25.8 |
|
|
Length of the OPLL (mm) |
|
|
0.798 |
|
0.499 |
|
0.554 |
|
ŌĆāŌēź6 |
14 |
6.6┬▒2.2 |
|
9.1┬▒1.7 |
|
58.0┬▒29.7 |
|
|
ŌĆā<6 |
11 |
6.8┬▒2.4 |
|
8.5┬▒2.0 |
|
50.0┬▒35.3 |
|
|
Severe cord compression level |
|
|
0.010 |
|
0.327 |
|
0.753 |
|
ŌĆāUpper |
9 |
7.2┬▒2.3 |
|
9.1┬▒1.8 |
|
58.8┬▒28.7 |
|
|
ŌĆāMiddle |
9 |
5.0┬▒1.7 |
|
8.1┬▒2.0 |
|
56.1┬▒27.2 |
|
|
ŌĆāLower |
7 |
8.1┬▒1.7 |
|
9.4┬▒1.5 |
|
46.7┬▒43.2 |
|
Table┬Ā3.
Surgical outcomes related with different approaches
|
Surgical approaches
|
p-value |
|
Anterior (n=7) |
Posterior (n=13) |
Combined (n=5) |
|
Pre Op. JOA score |
6.7┬▒2.6 |
6.8┬▒2.3 |
6.2┬▒2.3 |
0.875 |
|
Post Op. JOA score |
8.9┬▒2.1 |
9.0┬▒1.9 |
8.4┬▒1.5 |
0.837 |
|
Recovery rate |
59.5┬▒30.9 |
59.3┬▒33.5 |
34.8┬▒25.9 |
0.316 |
Table┬Ā4.
Surgical outcomes according to instrumentation about posterior approach
|
Instrumentation
|
p-value |
|
Yes (n=11) |
No (n=2) |
|
Pre Op. JOA score |
7.4┬▒2.1 |
4.0┬▒1.4 |
0.055 |
|
Post Op. JOA score |
9.5┬▒1.4 |
6.0┬▒1.4 |
0.008 |
|
Recovery rate |
64.1┬▒32.6 |
29.0┬▒5.66 |
0.174 |
Table┬Ā5.
Recovery rate according to dekyphosis
|
Approach |
Value |
Recovery rate |
p-value |
|
Dekyphosis |
|
|
|
|
ŌĆāYes (n=7) |
|
83.3┬▒23.6 |
0.047 |
|
ŌĆāŌĆāPosterior |
4 |
|
|
|
ŌĆāŌĆāAnterior |
1 |
|
|
|
ŌĆāŌĆāCombined |
2 |
|
|
|
ŌĆāNo (n=18) |
|
48.2┬▒29.4 |
|
|
ŌĆāŌĆāPosterior |
9 |
|
|
|
ŌĆāŌĆāAnterior |
6 |
|
|
|
ŌĆāŌĆāCombined |
3 |
|
|
Table┬Ā6.
PatientsŌĆÖ surgical outcomes according to degree of dekyphosis
|
Patients No. |
Age (years) |
Sex |
Fusion level |
CobbŌĆÖs angle (┬░)
|
JOA score
|
RR (%) |
|
Pre Op. |
Post Op. |
Change |
Pre Op. |
Final F/U |
|
1 |
51 |
Female |
T8-11 |
11.3 |
10.1 |
-1.2 |
8 |
11 |
100 |
|
2 |
40 |
Female |
T7-8 |
9.2 |
8.1 |
-1.1 |
8 |
11 |
100 |
|
3 |
54 |
Female |
T1-4 |
9.5 |
5.3 |
-4.2 |
5 |
10 |
83.3 |
|
4 |
48 |
Female |
T1-7 |
30.5 |
28.5 |
-2.0 |
5 |
8 |
50 |
Table┬Ā7.
Complication rates related with surgical approaches
|
Anterior (n=7) |
Posterior (n=13) |
Combined (n=5) |
p-value |
|
Complication |
3 (42.9) |
2 (15.4) |
3 (60) |
0.696 |
|
ŌĆāDural tear |
1 (14.3) |
1 (7.7) |
3 (60) |
0.088 |
|
ŌĆāPost Op. motor weakness |
2 (28.6) |
1 (7.7) |
1 (20) |
0.597 |
|
ŌĆāEpidural hematoma |
1 (14.3) |
1 (7.7) |
0 |
0.378 |
Table┬Ā8.
Complication rates according to dekyphosis
|
Dekyphosis
|
p-value |
|
Yes (n=7) |
No (n=18) |
|
Complications |
3 (42.9) |
5 (27.8) |
0.640 |
|
Dural tear |
2 (28.6) |
3 (16.7) |
0.597 |
|
Epidural hematoma |
0 (0) |
2 (11.1) |
1.000 |
|
Motor deterioration |
1 (14.3) |
3 (16.7) |
1.000 |
Table┬Ā9.
Recovery rate according to complication
|
Value |
Recovery rate |
p-value |
|
Complication |
|
|
0.857 |
|
ŌĆāYes |
8 |
52.6┬▒36.0 |
|
|
ŌĆāNo |
17 |
55.4┬▒30.8 |
|
|
ŌĆāDural tear |
|
|
0.698 |
|
ŌĆāŌĆāYes |
5 |
48.2┬▒40.0 |
|
|
ŌĆāŌĆāNo |
20 |
56.0┬▒30.5 |
|
|
ŌĆāPost Op. motor weakness |
|
|
0.737 |
|
ŌĆāŌĆāYes |
4 |
59.2┬▒28.4 |
|
|
ŌĆāŌĆāNo |
21 |
53.6┬▒33.0 |
|
|
ŌĆāEpidural hematoma |
|
|
0.632 |
|
ŌĆāŌĆāYes |
2 |
71.5┬▒40.3 |
|
|
ŌĆāŌĆāNo |
23 |
53.0┬▒31.7 |
|
References
1. Ando K, Imagama S, Kobayashi K, Hida T, Ito K, Tsushima M, et al : Comparative study of surgical treatment and nonsurgical follow up for thoracic ossification of the posterior longitudinal ligament: radiological and clinical evaluation. Spine (Phila Pa 1976) 42 : 407-410, 2017   2. Fujimura Y, Nishi Y, Nakamura M, Watanabe M, Matsumoto M : Myelopathy secondary to ossification of the posterior longitudinal ligament of the thoracic spine treated by anterior decompression and bony fusion. Spinal Cord 35 : 777-784, 1997    3. Hu P, Yu M, Liu X, Liu Z, Jiang L : A circumferential decompressionbased surgical strategy for multilevel ossification of thoracic posterior longitudinal ligament. Spine J 15 : 2484-2492, 2015   7. Imagama S, Ando K, Ito Z, Kobayashi K, Hida T, Ito K, et al : Risk factors for ineffectiveness of posterior decompression and dekyphotic corrective fusion with instrumentation for beak-type thoracic ossification of the posterior longitudinal ligament: a single institute study. Neurosurgery 80 : 800-808, 2017    8. Kawahara N, Tomita K, Murakami H, Hato T, Demura S, Sekino Y, et al : Circumspinal decompression with dekyphosis stabilization for thoracic myelopathy due to ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 33 : 39-46, 2008   9. Kim B, Yoon DH, Shin HC, Kim KN, Yi S, Shin DA, et al : Surgical outcome and prognostic factors of anterior decompression and fusion for cervical compressive myelopathy due to ossification of the posterior longitudinal ligament. Spine J 15 : 875-884, 2015   12. Koda M, Furuya T, Okawa A, Inada T, Kamiya K, Ota M, et al : Midto long-term outcomes of posterior decompression with instrumented fusion for thoracic ossification of the posterior longitudinal ligament. J Clin Neurosci 27 : 87-90, 2016   13. Komagata M, Inahata Y, Nishiyama M, Endo K, Tanaka H, Kobayashi H : Treatment of myelopathy due to cervicothoracic OPLL via open door laminoplasty. J Spinal Disord Tech 20 : 342-346, 2007   15. Matsumoto M, Chiba K, Toyama Y, Takeshita K, Seichi A, Nakamura K, et al : Surgical results and related factors for ossification of posterior longitudinal ligament of the thoracic spine: a multi-institutional retrospective study. Spine (Phila Pa 1976) 33 : 1034-1041, 2008   16. Matsumoto M, Toyama Y, Chikuda H, Takeshita K, Kato T, Shindo S, et al : Outcomes of fusion surgery for ossification of the posterior longitudinal ligament of the thoracic spine: a multicenter retrospective survey: clinical article. J Neurosurg Spine 15 : 380-385, 2011   17. Matsunaga S : Updates on ossification of posterior longitudinal ligament. Epidemiology and pathogenesis of OPLL. Clin Calcium 19 : 1415-1420, 2009  19. Matsuyama Y, Yoshihara H, Tsuji T, Sakai Y, Yukawa Y, Nakamura H, et al : Surgical outcome of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine: implication of the type of ossification and surgical options. J Spinal Disord Tech 18 : 492-497; discussion 498, 2005   20. Mori K, Imai S, Kasahara T, Nishizawa K, Mimura T, Matsusue Y : Prevalence, distribution, and morphology of thoracic ossification of the posterior longitudinal ligament in Japanese: results of CT-based crosssectional study. Spine (Phila Pa 1976) 39 : 394-399, 2014   21. Ohtsuka K, Terayama K, Yanagihara M, Wada K, Kasuga K, Machida T, et al : A radiological population study on the ossification of the posterior longitudinal ligament in the spine. Arch Orthop Trauma Surg 106 : 89-93, 1987    22. Shimomura T, Sumi M, Nishida K, Maeno K, Tadokoro K, Miyamoto H, et al : Prognostic factors for deterioration of patients with cervical spondylotic myelopathy after nonsurgical treatment. Spine (Phila Pa 1976) 32 : 2474-2479, 2007   24. Xu N, Yu M, Liu X, Sun C, Chen Z, Liu Z : A systematic review of complications in thoracic spine surgery for ossification of the posterior longitudinal ligament. Eur Spine J 26 : 1803-1809, 2017    25. Yamazaki M, Mochizuki M, Ikeda Y, Sodeyama T, Okawa A, Koda M, et al : Clinical results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament: operative indication of posterior decompression with instrumented fusion. Spine (Phila Pa 1976) 31 : 1452-1460, 2006   27. Zhang HQ, Chen LQ, Liu SH, Zhao D, Guo CF : Posterior decompression with kyphosis correction for thoracic myelopathy due to ossification of the ligamentum flavum and ossification of the posterior longitudinal ligament at the same level. J Neurosurg Spine 13 : 116-122, 2010  
|
|



















