Park, Son, Jung, Lee, and Yoo: Safety and Efficacy of Bone Cement (Spinofill®) for Vertebroplasty in Patients with Osteoporotic Compression Fracture : A Preliminary Prospective Study
Abstract
Objective
Although several commercialized bone cements are used during percutaneous vertebroplasty (PVP) for patients with osteoporotic vertebral compression fracture (OVCF), there are no reports using domestic products from South Korea. In this study, we investigated the safety and efficacy of Spinofill® (Injecta Inc., Gunpo, Korea), a new polymethyl methacrylate product.
Methods
A prospective, single-center, and single-arm clinical trial of 30 participants who underwent PVP using Spinofill┬« for painful thoracolumbar OVCF was performed with 6-months follow-up. Clinical and surgical outcomes included the Visual analog scale (VAS), Korean-Oswestry disability index (K-ODI), and OdomŌĆÖs criteria, complication rate, and recurrence rate. Radiological outcomes were evaluated by measuring the findings of postoperative computed tomography and simple radiograph.
Results
The pain of VAS (from 8.95┬▒1.05 to 4.65┬▒2.06, p<0.001) and the life quality based on K-ODI (from 33.95┬▒5.84 to 25.65┬▒4.79, p<0.001) improved significantly, and successful patient satisfaction were achieved in 20 patients (66.7%) 1 day after surgery. These immediate improvements were maintained or more improved during the follow-up. There was no surgery- or product-related complications, but OVCF recurred in two patients (6.7%). Favorable cement interdigitation was reported in 24 patients (80.0%), and extra-vertebral cement leakage was reported in 13 patients (43.0%). The mean vertebral height ratio (from 60.49%┬▒21.97% to 80.07%┬▒13.16%, p<0.001) and segmental kyphotic angle (from 11.46┬░┬▒8.50┬░ to 7.79┬░┬▒6.08┬░, p=0.002) improved one day after surgery. However, these short-term radiological findings somewhat regressed at the end.
Conclusion
The overall outcomes of PVP using Spinofill® were as favorable as those of other conventionally used products.
Key Words: Compression fracture ┬Ę Vertebra ┬Ę Vertebroplasty ┬Ę Bone cements ┬Ę Polymethyl methacrylate ┬Ę Spinofill.
INTRODUCTION
It is estimated that approximately 1.4 million new osteoporotic vertebral compression fracture (OVCF) occur in the elderly population every year worldwide [ 3, 13]. OVCF is related to severe pain, poor quality of life, and various complications due to a vicious cycle. Since the early era of percutaneous vertebroplasty (PVP) in the 1990s and approval of polymethyl methacrylate (PMMA) by US Food and Drug Administration in 2004, vertebroplasty using PMMA has been one of the standard treatments for pain control and early recovery of daily life [ 6, 9- 12, 18, 25]. Currently, many PMMA products by global companies have been used in clinical field, and their outcomes are favorable [ 2, 4, 16, 26, 29]. In Korea, due to the accumulation of clinical evidence of the safety and efficacy of PVP and advancement of surgical materials based on PMMA, PVP for OVCF has become popular since the 2000s [ 25]. However, as Korea is dependent on PMMA products from foreign companies, reports on domestic products are limited. Spinofill® (Injecta Inc., Gunpo, Korea), the most used product among several domestic products, was approved by the Korea Ministry of Food and Drug Safety in July 2013 and has been used in clinical practice since November 2013. In this prospective clinical trial, we investigated the safety and efficacy of Spinofill® in patients who underwent PVP for OVCF.
MATERIALS AND METHODS
Trial design and ethics
This was a single-center, single-arm, open-label trial with a 6-month follow-up ( Fig. 1). It was conducted in accordance with the 1964 Helsinki Declaration and its later amendments and was approved by the Institutional Review Board of Gil Medical Center (GDIRB2020-242). This research was registered as a clinical trial in the Clinical Research Information Service of Korea (KCT0005425).
Time frame
The final follow-up period was at 6 months, and the study was conducted over 18 months. In all patients, the hospital stay was three days, including the day before intervention, intervention day, and the day after intervention. Informed consent and baseline characteristics were obtained 1 day before intervention, and an assessment was performed in the inpatient ward 1 day after intervention. Afterwards, follow-up at 1 month and 6 months after procedure were done in all patients.
Aims
The primary endpoint was short-term and long-term efficacy based on the change in pain degree using the Visual analog scale (VAS). The secondary endpoint included improvement of quality of life, patient satisfaction, and change in radiological findings during the follow-up period. Safety was estimated by investigating any adverse events related to surgery or Spinofill®, cement leakage after PVP, and recurrence of OVCF during the follow-up period.
Sample size
The number of samples was calculated as follows :
Based on the results of previous clinical trials of PVP [ 4, 7, 8, 16, 18, 29], the standard deviation (Žā) of the main effect variable was set to 1, the non-inferiority limit (╬Ą) was set to 0.45, and the difference of effect (d) was set to 0. Using the above formula with a significance level of 5% and power of 80% (Z ╬▒=1.96 and Z ╬▓=0.842), the sample size of 30 patients was calculated. Considering a dropout rate of 20%, we recruited a total of 38 patients.
Indications and the patient population
Participants were recruited from the patients of neurosurgeons who specialized in spine disease and thoracolumbar OVCF. Acute thoracolumbar OVCF was confirmed by thoracic and lumbar spine radiography and magnetic resonance imaging (MRI). All potential participants were screened to determine eligibility according to the following inclusion and exclusion criteria based on the National Health Insurance guidelines.
The inclusion criteria were as follows : 1) adults aged >20 years with osteoporosis (Ōēż-2.5 of worst mean T-score of twolevel lumbar spine measured by dual energy X-ray absorptiometry) and single-level OVCF; 2) in patients aged <80 years, persistent severe back pain (VAS of pain level Ōēź7 and Korean version of Oswestry disability index (K-ODI) Ōēź20) despite conservative treatment for at least 2 weeks; 3) in patients aged Ōēź80 years, severe pain that makes daily life impossible (VAS of pain level Ōēź7 and K-ODI Ōēź20) regardless of the period of conservative treatment; and 4) those who voluntarily decided to participate in this clinical trial and were willing to comply with the clinical trial protocol.
The exclusion criteria were as follows : 1) patients with bleeding predisposition, infection, or metastatic cancer; 2) those with spinal canal involvement that causes neurological symptoms including paralysis or urinary/defecation abnormality; 3) hypersensitivity to PMMA components; 4) pain disorders other than OVCF, such as herpes zoster syndrome or fibromyalgia; 5) history of previous surgery on the thoracolumbar spine; 6) pregnant or lactating women and women with childbearing potential who plan to become pregnant; and 7) those inappropriate to participate in this clinical trial according to the judgment of the researchers.
Surgical procedure
Two surgeons performed the same sequence of PVP according to the general surgical technique. After the patient was placed in a prone position on a radiolucent bed, local anesthesia and light sedation were introduced. After insertion of a 10-G bone marrow needle into the targeted location of the vertebral body via a bilateral transpedicular approach under fluoroscopic guidance, Spinofill ® was mixed with a dedicated lysate in a disposable container included in the instrument set to prepare the injection. After the PMMA hardened like a toothpaste, approximately 5 minutes after mixing, PMMA was injected using a 1-mL syringe until satisfactory filling and distribution in the vertebral body were achieved [ 27]. The volume of the inserted PMMA was decided according to the literature and judgment of the surgeon during the procedure [ 14, 20, 21]. Finally, a one-point skin suture using nylon 3-0 was done on each side.
Medical treatment
All patients received the usual care for patients who underwent PVP in the inpatient ward and outpatient clinic. Medication for pain control was unrestricted following the discretion of the patient and surgeon, and all analgesia given were recorded at every follow-up assessment. Furthermore, osteoporosis management was decided based on various strategies according to the patientŌĆÖs circumstances and compliance, as well as the surgeonŌĆÖs recommendation based on general guidelines [ 5, 24, 28, 32].
Outcome assessment
The same blinded investigator evaluated all patients at initially and during the follow-up period. Demographic data, including age and sex; as well as baseline characteristics, including body mass index, past medical history, worst mean T-score of two-level lumbar spine according to dual energy X-ray absorptiometry, surgery level, and injected cement volume during surgery were recorded.
Clinical and radiological data were collected a day before surgery, a day after surgery, 1 month ┬▒1 week after surgery, and 6 months ┬▒2 weeks after surgery in all patients.
The degree of back pain was evaluated using VAS scores with a range of 0-10 points. Health-related quality of life was assessed using the K-ODI with a range of 0-100% [ 15]. Patient satisfaction was assessed using OdomŌĆÖs criteria after surgery [ 23]. Simple radiographs (standing anteroposterior view and lateral view) and thoracolumbar MRI were performed prior to surgery, and simple radiography and computed tomography (CT) were performed immediately after surgery to analyze cement interdigitation and cement leakage. Simple radiography was performed at each follow-up visit. The degree of interdigitation of bone cement on the postoperative CT was scored on semiquantitative scale with a range of grades 1-4 using the classification proposed by Nieuwenhuijse et al. [ 22] : grade 1, complete interdigitation throughout the injected volume with clearly visible bone trabeculae; grade 2, considerable interdigitation along the boundaries of a clearly recognizable cement deposit; grade 3, a clump like filling pattern with sparsely gross interdigitation; and grade 4, no interdigitation at all with sharp boundaries along the cement clump, comparable to cleft filling. Also, the presence of any extra-vertebral cement leakage was assessed on postoperative CT and classified according to site of leakage ( Fig. 2) [ 25]. The vertebral height ratio and segmental kyphotic angle of surgery level (measured using CobbŌĆÖs method) on standing lateral radiographs in a neutral position were used to evaluate the progression of vertebral collapse ( Fig. 3) [ 17]. Two independent investigators measured the same image twice for all radiological parameters. If there was any disagreement about the qualitative parameters between the researchers, a conclusion was reached by consensus. The quantitative parameter was determined as the average of the results of the two researchers. Surgery-related complications, such as neurologic deterioration, wound infection, and embolism were observed carefully after the surgery. Furthermore, recurrence of OVCF at another level was screened by regular simple radiography and/or pain aggravation during follow-up.
Statistical analysis
Data management and statistical analyses were performed using SPSS (version 27.0; IBM Corporation, Armonk, NY, USA). CohenŌĆÖs kappa and intraclass coefficient correlation (ICC) were used to assess both intra-observer and inter-observer reproducibility. One-way analysis of variance (ANOVA), paired t-test, PearsonŌĆÖs chi square test, and Kaplan-Meier survival analysis were used according to the characteristics of the factors.
The results were expressed as mean┬▒standard deviation, mean and corresponding 95% confidence interval (CI), or medians with ranges depending on whether the data were normally distributed. Statistical significance was accepted for p-values <0.05.
RESULTS
Demographic data and baseline characteristics
Among the registered 38 patients, eight patients were excluded (two patients with simple consent withdrawal, four patients with any loss of fixed follow-up visit, one patient died from underlying cardiovascular disease, and one patient died from underlying cancer). Finally, 30 patients were enrolled in the study design. The 30 study participants comprised seven men and 23 women, with an overall mean age of 78.3┬▒8.3 years. The mean body mass index was 22.9┬▒3.2, and median worst T-score was -3.3 (range, -5.1 to -2.5). The median duration from onset to surgery was 13.0 days (range, 1.0 to 43.0), and median duration from diagnosis using MRI to surgery was 7.0 days (range, 1.0 to 43.0).
Surgery levels were T11 in one patient, T12 in seven patients, L1 in 12 patients, L2 in two patients, above T11 in two patients, and below L2 in six patients.
The median injected cement volume was 5.0 mL (range, 2.5 to 6.0). Various underlying diseases were observed in 27 patients (90%). The most common comorbidities were cardiovascular disease, including hypertension and other heart disease, and pervious osteoporotic fracture were in 11 patients (36.7%) ( Table 1).
Clinical outcomes
The mean preoperative VAS for back pain was 8.95┬▒1.05, and this decreased to 4.65┬▒2.06 at 1-day post-operation, 3.35┬▒ 2.18 at 1-month post-operation, and 4.20┬▒3.07 at 6-month post-operation ( p<0.001, one-way ANOVA). The pre-operation to 1-day post-operation, pre-operation to 1-month post-operation, and pre-operation to 6-month post-operation difference in back VAS scores were significant (the mean difference 4.17 [95% CI, 3.36 to 4.97], 5.68 [95% CI, 4.72 to 6.63], and 4.90 [95% CI, 3.60 to 6.21], respectively, p<0.001, paired t-test), but the difference during follow-up after operation was not significant. Although the 1-month post-operation to 6-month post-operation difference was not significant, the 1-day post-operation to 1-month post-operation difference in back VAS scores were significant (the mean difference 1.64 [95% CI, 0.51 to 2.78], p=0.006, paired t-test) ( Table 2). The mean K-ODIs improved significantly from 33.95┬▒5.84 at pre-operation to 25.65┬▒4.79 at 1-day post-operation, 20.85┬▒7.75 at 1-month post-operation, and 19.55┬▒11.89 at 6-month post-operation ( p<0.001, one-way ANOVA). The pre-operation to 1-day post-operation, pre-operation to 1-month post-operation, and pre-operation to 6-month post-operation difference in K-ODI were significant (the mean difference 7.56 [95% CI, 5.52 to 9.61], 12.25 [95% CI, 8.55 to 15.95], and 14.33 [95% CI, 8.29 to 20.38], respectively, p<0.001, paired t-test). Although the 1-month post-operation to 6-month post-operation difference was not significant, the 1-day post-operation to 1-month post-operation difference in K-ODI was significant (the mean difference 4.71 [95% CI, 1.71 to 7.72], p=0.003, paired t-test) ( Table 2). According to OdomŌĆÖs criteria, the success rate, including excellent or good outcomes, were 66.7% at 1-day post-operation, 83.3% at 1-month post-operation, and 80.0% at 6-month post-operation. OdomŌĆÖs criteria distributions at the three time points of after surgery were not significantly different ( p=0.208 and 0.458, PearsonŌĆÖs chi square test) ( Table 2).
Radiological outcomes
The measurements of qualitative postoperative CT findings had high intra- and inter-observer reliabilities ranging between 0.924 and 0.965 based on CohenŌĆÖs kappa, and the measurement of quantitative simple radiograph findings had high intra- and inter-observer reliabilities ranging between 0.852 and 0.943 based on ICC.
Cement interdigitation was classified as grade 1 in 10 patients, grade 2 in 14 patients, grade 3 in five patients, and grade 4 in one patient ( Table 2). Extra-vertebral cement leakage occurred in 13 patients (43.0%) with the following distributions : type I (epidural leakage) in four patients, type II (cortical leakage) in one patient, type III (disc space leakage) in four patients, type IV vascular leakage in two patients, and mixed type in two patients (one patient with type I and III, and one patient with type III and IV) ( Table 3). However, there were no clinical events related to cement leakage or cement interdigitation. For all the 30 study participants, the mean vertebral height ratio at surgical levels increased significantly from 60.49%┬▒21.97% preoperatively to 80.07%┬▒13.16% at 1-day post-operation (the mean difference 16.19% [95% CI, 21.93 to 10.44], p<0.001, paired t-test). However, it decreased to 74.34%┬▒14.77% at 1-month post-operation (the mean difference 6.61% [95% CI, 3.00 to 10.23], p<0.001, paired t-test), and remained the same at 6-month post-operation ( Table 3). Similar to the vertebral height ratio, the mean segmental kyphotic angles at surgical levels were significantly improved from 11.46┬░┬▒8.50┬░ preoperatively to 7.79┬░┬▒6.08┬░ at 1-day postoperation (the mean difference 2.94┬░ [95% CI, 1.16 to 4.72], p=0.002, paired t-test). However, it increased to 9.88┬░┬▒7.35┬░ at 1-month post-operation (the mean difference 1.89┬░ [95% CI, 2.97 to 0.81], p<0.001, paired t-test) ( Table 3).
Surgical outcomes
Except for two cases of trivial postoperative complications (one case of diarrhea and one case of ileus), no major surgical complications, such as neurologic aggravation, wound infection, or cement embolism, were encountered. In addition, with except for one case of cerebral infarction at 2 months after surgery in a 76-year-old patient, no perioperative morbidity related to surgery, such as cardiopulmonary problems or deep vein thrombosis, occurred.
Two patients experienced recurrence of OVCF at another vertebral body (one patient recurred at L1 after 14 days of PVP L5 and one patient recurred at L1 after 84 days of PVP L4). According to Kaplan-Meier survival analysis, the 6-month recurrence-free survival rate was 93.3% and the estimated overall time to a recurrence was 171.27 days (95% CI, 159.13 to 183.40) ( Fig. 4).
Medical treatment
All patients received the usual medications for pain control, including acetaminophen, non-steroidal anti-inflammatory drugs, and tramadol. In terms of osteoporosis management, eight patients (26.7%) received zoledronic acid, 10 patients (33.3%) received denosumab, and 12 patients (40.0%) received no treatment due to the patientŌĆÖs discretion and circumstances. There was no statistically significant difference between osteoporosis management and recurrence of OVCF because of the small sample size.
DISCUSSION
Spinofill ┬« is composed of PMMA powder and a solution of methyl methacrylate with barium, similar to other existing products but the proportions of each component are slightly different. According to a biomechanical study of the company, the ideal working time following mixing was 15.5 minutes at 23Ōäā of environment. Working time may differ depending on the temperature of use and storage environment. The maximum temperature of material after mixing is below 75Ōäā, which is similar or safter than other products [ 11]. Although the bending modulus is similar to that of other products, the bending strength and compressive strength are better compared to those of other previous materials [ 11]. In addition, the instrument set includes a sterilized disposable bowl and spatula for the mixing process to reduce infection and hassle during surgery ( Fig. 5). As expected, the measures of clinical outcome, including VAS score and K-ODI, showed significant improvement immediately after surgery (4.17 [95% CI, 3.36 to 4.97) of VAS improvement and 7.56 [95% CI, 5.52 to 9.61] of K-ODI improvement) and further improvement at 1-month after surgery (1.64 [95% CI, 0.51 to 2.78] of VAS improvement and 4.71 [95% CI, 1.71 to 7.72] of K-ODI improvement). In addition, in terms of patient satisfaction, success rate of surgery was 66.7% at 1-day after operation and reached 83.3% at 1-month post-operation. However, clinical improvements ceased after 1-month postoperatively. This phenomenon may occur due to the apparent effect of surgery during the short-term period and less apparent effect in the long-term period. The favorable immediate clinical outcome and plateau during mid-term follow-up were similar the most previous reports about short- or mid-term outcomes after PVP [ 7, 10, 16, 25, 26]. Based on these findings, the efficacy associated with the clinical effect is comparable to that of other PMMA products. The distribution of cement interdigitation with 80% favorable interdigitation was not significantly different from that in previous studies, ranging from 51.8% to 95.6% [ 22, 25]. Moreover, the occurrence of cement leakage (43.0%), including four patients (13.3%) with epidural leakage, was not significantly different from that in previous studies, ranging from 18.4% to 54.7% [ 18, 30, 31]. Furthermore, there were no clinical symptoms related to cement interdigitation or cement leakage. Based on these findings, the safety associated with the injection and distribution of the material during surgery is proven. Although the vertebral height ratio and segmental kyphotic angle improved immediately after surgery, these improvements were reduced again over the course of 6 months. This phenomenon is comparable with the results of previous studies due to the spontaneous natural course of collapse after occurrence of OVCF [ 2, 7, 25, 29]. Based on these findings, the efficacy associated with the radiological results is reasonable compared with other PMMA products. Based on the low incidence of surgery-related complications and absence of serious adverse events, the safety associated with material-related complications was preliminarily proven. In the recurrence rate after PVP in this study of 6.7% was acceptable compared to previous reports, ranging from 6.2% to 52.8% [ 1, 19]. Because the follow-up period was mid-term (6 months) in this study, the incidence of new vertebral fractures might appear to be low compared with previous studies. Based on these findings, the safety associated with product-related complications and recurrence of new fractures was verified. The present study has several limitations. First, because of its single-arm design, direct comparisons with other products were not possible. However, based on previous clinical trials, we could indirectly compare between Spinofill┬« with other products. Second, the number of enrolled patients was small. Nonetheless, based on the purpose of this study as a preliminary demonstration of non-inferiority compared to other products in the market, the study has a sufficient sample size.
Almost all the results of this study were consistent with previous literature and did not deviate from what was expected. However, we believed that the results obtained are valuable because this is the first study on the domestic product of PMMA in Korea. We suggest that further large-scale, randomized controlled trials for direct comparison between Spinofill® and other major products should be conducted to confirm our results.
CONCLUSION
This prospective clinical trial showed that Spinofill® is an effective and safe PMMA product for PVP in patients with OVCF. Based on the results of this study, although further clinical investigation is necessary, Spinofill® has the potential to be used safely in the clinical field.
Acknowledgements
This research was supported by a grant from the Gachon University Gil Medical Center (grant No. FRD2020-15) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021M3I2A1077405).
Fig.┬Ā1.
Diagram of recruitment and study process. 
Fig.┬Ā2.
Classification of cement leakage : type I, epidural leakage via the basivertebral vein or fracture defect; type II, cortical leakage through cortical defect; type III, disc space leakage through end plate defect; and type IV, vascular leakage via the segmental vein. 
Fig.┬Ā3.
Lateral plain radiograph showing measured factors. The mean height of the vertebral body was defined as the average of the three area heights of the vertebral body ([a+b+c] / 3). The vertebral height ratio was calculated using the following equation : the vertebral height ratio (%) = mean height of the index level / [(mean height of the upper adjacent body + mean height of the lower adjacent body) / 2] ├Ś 100. Segmental kyphotic angle (X) was determined at the intersection of lines drawn at the superior plateau of vertebral body and the inferior plateau of vertebral body. 
Fig.┬Ā4.
Kaplan-Meier analysis of recurrence-free survival rate. 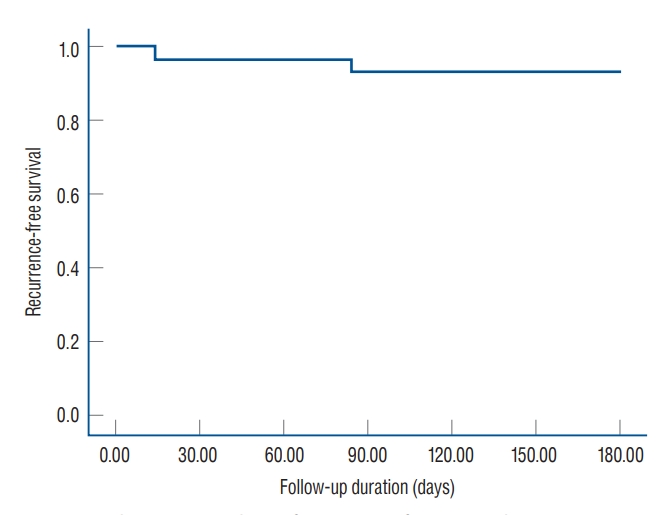
Fig.┬Ā5.
Composition of Spinofill® (Injecta Inc., Gunpo, Korea). 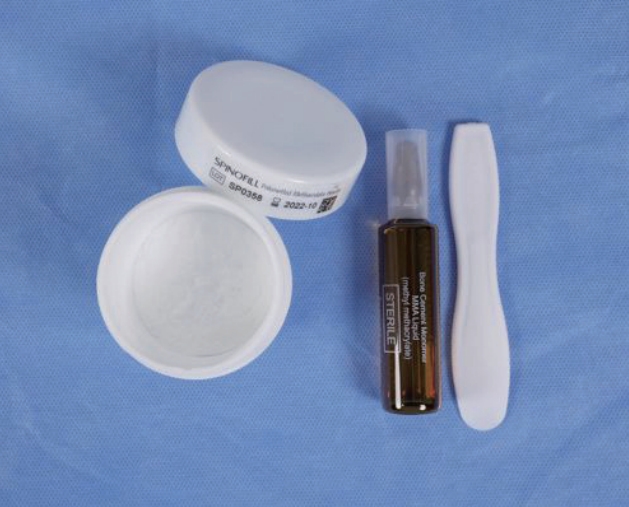
Table┬Ā1.
Demographic data and baseline characteristics
|
Value (n=30) |
|
Age (years) |
78.3┬▒8.3 |
|
Men/women |
7/23 |
|
Body mass index (kg/m2) |
22.9┬▒3.2 |
|
Worst T-score of bone mineral density |
-3.3 (-5.1 to -2.5) |
|
Median duration from onset to surgery (days) |
13.0 (1.0 to 43.0) |
|
Median duration from diagnosis to surgery (days) |
7.0 (1.0 to 43.0) |
|
Surgeon |
|
|
ŌĆāA |
26 |
|
ŌĆāB |
4 |
|
Surgery level |
|
|
ŌĆāT11 |
1 |
|
ŌĆāT12 |
7 |
|
ŌĆāL1 |
12 |
|
ŌĆāL2 |
2 |
|
ŌĆāAbove T11 |
2 |
|
ŌĆāBelow L2 |
6 |
|
Injected cement volume (mL) |
5.0 (2.5 to 6.0) |
|
Comorbidities |
|
|
ŌĆāHypertension |
25 |
|
ŌĆāOther heart disease |
16 |
|
ŌĆāCerebrovascular disease |
11 |
|
ŌĆāDiabetes |
10 |
|
ŌĆāCancer |
10 |
|
ŌĆāPulmonary disease |
3 |
|
ŌĆāLiver disease |
3 |
|
ŌĆāKidney disease |
3 |
|
ŌĆāPrevious fracture history |
11 |
Table┬Ā2.
|
Characteristic |
Value |
p-value |
|
VAS back |
|
<0.001*
|
|
ŌĆā |
Pre OP |
8.95┬▒1.05 |
|
|
1-day |
4.65┬▒2.06 |
|
|
1-month |
3.35┬▒2.18 |
|
|
6-month |
4.20┬▒3.07 |
|
|
ΔVAS back |
|
|
|
Pre OP to 1-day |
4.17 (3.36 to 4.97) |
<0.001ŌĆĀ
|
|
Pre OP to 1-month |
5.68 (4.72 to 6.63) |
<0.001ŌĆĀ
|
|
Pre OP to 6-month |
4.90 (3.60 to 6.21) |
<0.001ŌĆĀ
|
|
1-day to 1-month |
1.64 (0.51 to 2.78) |
0.006ŌĆĀ
|
|
1-day to 6-month |
2.46 (-0.60 to 1.64) |
0.341ŌĆĀ
|
|
1-month to 6-month |
-0.85 (-2.47 to -1.10) |
0.285ŌĆĀ
|
|
K-ODI |
|
<0.001*
|
|
Pre OP |
33.95┬▒5.84 |
|
|
1-day |
25.65┬▒4.79 |
|
|
1-month |
20.85┬▒7.75 |
|
|
6-month |
19.55┬▒11.89 |
|
|
ΔK-ODI |
|
|
|
Pre OP to 1-day |
7.56 (5.52 to 9.61) |
<0.001ŌĆĀ
|
|
Pre OP to 1-month |
12.25 (8.55 to 15.95) |
<0.001ŌĆĀ
|
|
Pre OP to 6-month |
14.33 (8.29 to 20.38) |
<0.001ŌĆĀ
|
|
1-day to 1-month |
4.71 (1.71 to 7.72) |
0.003ŌĆĀ
|
|
1-day to 6-month |
5.90 (0.84 to 10.97) |
0.025ŌĆĀ
|
|
1-month to 6-month |
1.30 (-2.86 to 5.46) |
0.521ŌĆĀ
|
|
OdomŌĆÖs criteria |
|
|
|
1-day; excellent/good/fair/poor |
1/19/9/1 |
|
|
1-month; excellent/good/fair/poor |
6/19/3/2 |
0.458ŌĆĪ
|
|
6-month; excellent/good/fair/poor |
8/16/5/1 |
0.208ŌĆĪ
|
|
Success rate at 1-day |
20 (66.7) |
|
|
Success rate at 1-month |
25 (83.3) |
|
|
Success rate at 6-month |
24 (80.0) |
|
Table┬Ā3.
|
Characteristic |
Value |
p-value |
|
Cement interdigitation |
|
|
|
ŌĆāGrade 1 |
10 (33.3) |
|
|
ŌĆāGrade 2 |
14 (46.7) |
|
|
ŌĆāGrade 3 |
5 (16.7) |
|
|
ŌĆāGrade 4 |
1 (3.3) |
|
|
Cement leakage |
13 (43.0) |
|
|
ŌĆāType I |
4 (13.3) |
|
|
ŌĆāType II |
1 (3.3) |
|
|
ŌĆāType III |
4 (13.3) |
|
|
ŌĆāType IV |
2 (6.7) |
|
|
ŌĆāMixed |
2 (6.7) |
|
|
Vertebral height ratio (%) |
|
<0.001*
|
|
ŌĆāPre OP |
60.49┬▒21.97 |
|
|
ŌĆā1-day |
80.07┬▒13.16 |
|
|
ŌĆā1-month |
74.34┬▒14.77 |
|
|
ŌĆā6-month |
75.73┬▒15.70 |
|
|
ΔVertebral height ratio (%) |
|
|
|
ŌĆāPre OP to 1-day |
-16.19 (-21.93 to -10.44) |
<0.001ŌĆĀ
|
|
ŌĆāPre OP to 1-month |
-7.93 (-16.69 to -2.76) |
0.008ŌĆĀ
|
|
ŌĆāPre OP to 6-month |
-13.49 (-24.19 to -2.78) |
0.016 |
|
ŌĆā1-day to 1-month |
6.61 (3.00 to 10.23) |
<0.001ŌĆĀ
|
|
ŌĆā1-day to 6-month |
4.34 (-1.10 to 9.79) |
0.112 |
|
ŌĆā1-month to 6-month |
-1.04 (-6.56 to 4.49) |
0.697 |
|
Segmental kyphotic angle (┬░) |
|
<0.001*
|
|
ŌĆāPre OP |
11.46┬▒8.50 |
|
|
ŌĆā1-day |
7.79┬▒6.08 |
|
|
ŌĆā1-month |
9.88┬▒7.35 |
|
|
ŌĆā6-month |
10.73┬▒6.85 |
|
|
ΔSegmental kyphotic angle (°) |
|
|
|
ŌĆāPre OP to 1-day |
2.94 (1.16 to 4.72) |
0.002ŌĆĀ
|
|
ŌĆāPre OP to 1-month |
0.99 (-1.12 to 3.10) |
0.343ŌĆĀ
|
|
ŌĆāPre OP to 6-month |
0.91 (-1.29 to 3.11) |
0.396ŌĆĀ
|
|
ŌĆā1-day to 1-month |
-1.89 (-2.97 to -0.81) |
0.001ŌĆĀ
|
|
ŌĆā1-day to 6-month |
-2.73 (-7.32 to -1.12) |
0.002ŌĆĀ
|
|
ŌĆā1-month to 6-month |
-0.86 (-1.93 to 0.21) |
0.106ŌĆĀ
|
References
1. Bae JS, Park JH, Kim KJ, Kim HS, Jang IT : Analysis of risk factors for secondary new vertebral compression fracture following percutaneous vertebroplasty in patients with osteoporosis. World Neurosurg 99 : 387-394, 2017   2. Balkarli H, Kilic M, Balkarli A, Erdogan M : An evaluation of the functional and radiological results of percutaneous vertebroplasty versus conservative treatment for acute symptomatic osteoporotic spinal fractures. Injury 47 : 865-871, 2016   3. Borgstr├Čm F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, et al : Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 17 : 637-650, 2006    6. Deramond H, Depriester C, Toussaint P : Vertebroplasty and percutaneous interventional radiology in bone metastases: techniques, indications, contra-indications. Bull Cancer Radiother 83 : 277-282, 1996  8. Firanescu CE, de Vries J, Lodder P, Venmans A, Schoemaker MC, Smeets AJ, et al : Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial. BMJ 361 : k1551, 2018    9. Gangi A, Kastler BA, Dietemann JL : Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR Am J Neuroradiol 15 : 83-86, 1994   10. Guo JB, Zhu Y, Chen BL, Xie B, Zhang WY, Yang YJ, et al : Surgical versus non-surgical treatment for vertebral compression fracture with osteopenia: a systematic review and meta-analysis. PLoS One 10 : e01271452015    12. Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE : Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol 18 : 1897-1904, 1997   13. Johnell O, Kanis JA : An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17 : 1726-1733, 2006    14. Kaufmann TJ, Trout AT, Kallmes DF : The effects of cement volume on clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol 27 : 1933-1937, 2006   15. Kim DY, Lee SH, Lee HY, Lee HJ, Chang SB, Chung SK, et al : Validation of the Korean version of the oswestry disability index. Spine (Phila Pa 1976) 30 : E123-E127, 2005   17. Klezl Z, Majeed H, Bommireddy R, John J : Early results after vertebral body stenting for fractures of the anterior column of the thoracolumbar spine. Injury 42 : 1038-1042, 2011   18. Lou S, Shi X, Zhang X, Lyu H, Li Z, Wang Y : Percutaneous vertebroplasty versus non-operative treatment for osteoporotic vertebral compression fractures: a meta-analysis of randomized controlled trials. Osteoporos Int 30 : 2369-2380, 2019    19. Ma X, Xing D, Ma J, Wang J, Chen Y, Xu W, et al : Risk factors for new vertebral compression fractures after percutaneous vertebroplasty: qualitative evidence synthesized from a systematic review. Spine (Phila Pa 1976) 38 : E713-E722, 2013  20. Martin─Źi─Ź D, Brojan M, Kosel F, ┼Ātern D, Vrtovec T, Antoli─Ź V, et al : Minimum cement volume for vertebroplasty. Int Orthop 39 : 727-733, 2015    21. Molloy S, Mathis JM, Belkoff SM : The effect of vertebral body percentage fill on mechanical behavior during percutaneous vertebroplasty. Spine (Phila Pa 1976) 28 : 1549-1554, 2003   22. Nieuwenhuijse MJ, Muijs SP, van Erkel AR, Dijkstra SP : A clinical comparative study on low versus medium viscosity polymethylmetacrylate bone cement in percutaneous vertebroplasty: viscosity associated with cement leakage. Spine (Phila Pa 1976) 35 : E1037-E1044, 2010  23. Odom GL, Finney W, Woodhall B : Cervical disk lesions. J Am Med Assoc 166 : 23-28, 1958   24. Qaseem A, Forciea MA, McLean RM, Denberg TD, Barry MJ, Cooke M, et al : Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American college of physicians. Ann Intern Med 166 : 818-839, 2017   25. Son S, Lee SG, Kim WK, Park CW, Yoo CJ : Early vertebroplasty versus delayed vertebroplasty for acute osteoporotic compression fracture : are the results of the two surgical strategies the same? J Korean Neurosurg Soc 56 : 211-217, 2014    26. Voormolen MH, Mali WP, Lohle PN, Fransen H, Lampmann LE, van der Graaf Y, et al : Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol 28 : 555-560, 2007   27. Wang M, Zhang L, Fu Z, Wang H, Wu Y : Selections of bone cement viscosity and volume in percutaneous vertebroplasty: a retrospective cohort study. World Neurosurg 150 : e218-e227, 2021   28. Watts NB, Camacho PM, Lewiecki EM, Petak SM, AACE/ACE Postmenopausal Osteoporosis Guidelines Task Force : American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract 27 : 379-380, 2021   29. Yang EZ, Xu JG, Huang GZ, Xiao WZ, Liu XK, Zeng BF, et al : Percutaneous vertebroplasty versus conservative treatment in aged patients with acute osteoporotic vertebral compression fractures: a prospective randomized controlled clinical study. Spine (Phila Pa 1976) 41 : 653-660, 2016  30. Zhan Y, Jiang J, Liao H, Tan H, Yang K : Risk factors for cement leakage after vertebroplasty or kyphoplasty: a meta-analysis of published evidence. World Neurosurg 101 : 633-642, 2017   32. Prevention and management of osteoporosis. World Health Organ Tech Rep Ser 921 : 1-164; backcover, 2003 
|
|





















