Han, Kim, Jeong, Lee, Chang, Park, and Kim: Electrophysiological and Behavioral Changes by Phosphodiesterase 4 Inhibitor in a Rat Model of Alcoholic Neuropathy
Abstract
Objective
Alcoholic neuropathy is characterized by allodynia (a discomfort evoked by normally innocuous stimuli), hyperalgesia (an exaggerated pain in response to painful stimuli) and spontaneous burning pain. The aim of the present study is to investigate the effect of rolipram, a phosphodiesterase 4 inhibitor, against alcohol-induced neuropathy in rats.
Methods
Allodynia was induced by administering 35% v/v ethanol (10 g/kg; oral gavage) to Spraue-Dawley rats for 8 weeks. Rolipram and saline (vehicle) were administered intraperitoneally. Mechanical allodynia was measured by using von Frey filaments. Somatosensory evoked potential (SEP) was proposed as complementary measure to assess the integrity of nerve pathway.
Results
The ethanol-induced mechanical allodynia began to manifest from 3 week, and then peaked within 1 week. Beginning from 3 week, latency significantly started to increased in control group. In rolipram treated rats, the shorter latency was sustained until 8 weeks (p<0.05). The mechanical allodynia, which began to manifest on the 3 weeks, intraperitoneal injections of rolipram sustained statistical difference until 8 weeks, the final week of the study (p<0.05).
Conclusion
This study suggests that rolipram might alleviate mechanical allodynia induced by alcohol in rats, which clearly has clinical implication.
Key Words: Alcoholic neuropathy · Phosphodiesterase 4 inhibitor · Rolipram.
INTRODUCTION
Polyneuropathy is a frequent complication of chronic consumption of ethanol, characterized by allodynia and pain, primarily in the lower extremities, and is poorly managed by available treatments 22). It is often asymptomatic, and incidence of peripheral neuropathy ranges from 10% to 50% 21). The mechanism of alcohol-induced neuropathy is an axonal neuropathy characterized by Wallerian degeneration of the axons and a reduction in the myelination of nerve fibers 34). The pathogenesis of this axonal degeneration and reduction in the myelination, however, is not well understood. Researches on animal models have suggested that alcohol has a direct toxic effect on peripheral nerve and the spinal cord system 5,23). Especially acetaldehyde, one of the most important metabolites of ethanol, has a direct neurotoxic effect 16). Epigenetic and inflammatory control is critical role in the experimental animal models of alcohol induced neuropathy. In the recent year, similar results have been obtained that protein kinase species play a critical role in the development and maintenance of alcohol-induced pain 6). Extensive animal study suggested that chronic administration of minocycline could ameliorate the development of neuropathic pain by inhibiting the release of proinflammatory cytokines and oxidative stress in mononeuropathic rats 7). A significant increase in lipid peroxidation and a significant decrease in the activity of antioxidant enzymes (superoxide dismutase and catalase) were observed in the sciatic nerves of diabetic rats with established neuropathic pain 28). Dina et al. 6) demonstrated that allodynia is present in an established rat model of chronic alcoholism and that an inflammatory process and protein kinase signaling play a pivotal role in the enhanced allodynia produced by chronic alcohol. An important action of cAMP is activation of transcription factors, including cAMP-responsive element binding (CREB) protein and nuclear factor-kB (NF-κB) p50 12). Phosphorylation of CREB stimulates transcription of cell survival genes 19). Phosphorylation of NF-κB p50 subunit suppresses transcription of inflammation-associated genes, especially proinflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-Iβ) 4,12,31). Thus, we hypothesized that use of rolipram, a selective inhibitor of cAMP specific phosphodiesterase (PDE), may improve mechanical allodynia and nerve conduction in a rat model of ethanol-induced neuropathic pain.
MATERIALS AND METHODS
Experimental animals
Male adult Sprague-Dawley rats weighing 200-300 g were used in this study. The animals were housed in groups of two in plastic cages with soft bedding and free access to food and water. All animals were acclimated to their cages for 1 week before any experiments were performed. All experimental protocols were approved by the Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Ethanol-induced neuropathic pain model and drug treatment protocol
Alcoholic neuropathy was induced by administering 10 g/kg b.i.d oral gavage of 35% v/v ethanol in double distilled water for 8 weeks in all animals. Rolipram (Sigma, St. Louis, MO, USA), 3 mg/kg was first dissolved in 75 µL and then gently mixed with 75 µL Tween 80 and 1850 µL physiological saline to a final 2,000 µL solution. Physiological saline served as the vehicle for the control group. According to the randomly assigned group, animals were administered rolipram or vehicle once daily for a period of eight weeks. 250 µL rolipram or vehicle was administered intraperitoneally. Intraperitoneal delivery involved an injection below the skin on the low abdominal region of the animal at the right flank.
Electrophysiologic responses
A Nicolet Viking IV was used for recording the somatosensory evoked potential (SEP). All SEP responses were obtained from stimulation of anesthetized, unrestrained rats. Rats were placed in a prone position on a plastic board with the active electrode implanted 2.5 mm posterior to the bregma and the reference electrode implanted in the mid frontal bone ( Fig. 1). The SEP responses were elicited by activating the sensory nerve in the tale. For the sensory nerve stimulation, the cathode was placed 4 cm distal to the tale origin site and the anode was positioned two fingers distal to the cathode with ring electrode. The ground electrode was placed subcutaneously between the stimulation and recording electrodes. The tail was stimulated by positive 1.3-1.9 mA current pulses for 0.2 msec at 3 Hz. The signal-to-noise ratio was improved by ensemble averaging of 500 stimulus locked sweeps. The first peak latency was recorded. All animals were evaluated once a week for 8 weeks.
Quantification for mechanical allodynia
The behavioral tests measured were foot withdrawal thresholds (as an indicator for mechanical allodynia) in response to mechanical stimuli applied to the left and right hind paws 22). The examiner who conducted the tests did not know about the nature of the experimental treatment. For each test, the animals were placed in a plastic chamber (9×9×30 cm) and habituated for at least 10 min. The chamber was placed on a mesh screen, so that mechanical stimuli could be administered to the plantar surface of the left and right hind paws. Thresholds were determined by the up-down method 7) by using a set of von Frey monofilaments (von Frey filament values: 3.65, 3.87, 4.10, 4.31, 4.52, 4.74, 4.92, and 5.16; equivalent to: 0.45, 0.74, 1.26, 2.04, 3.31, 5.50, 8.32, and 14.45 g values). A von Frey filament was applied perpendicularly to the most sensitive areas of the plantar surface at the base of the third or fourth toes with sufficient force to bend the filament slightly for 3-4 sec. An abrupt withdrawal of the foot during stimulation or immediately after stimulus removal was considered as a positive response. The first stimulus was always the 4.31 filament. When there was a positive response, a filament with the next lower von Frey value was used. When no response was observed, a filament with the next higher von Frey value was applied. This testing pattern continued until responses to the sixth von Frey stimuli from the first change of response (either higher or lower than the first stimulus depending on whether the first response was negative or positive) were measured. The responses were then converted into a 50% threshold value using the formula: 50% threshold 10 ( X
kd)/104, where X is the value of the final von Frey hair used in log units, k is the tabular value for the pattern of positive or negative responses, and d is the mean differences between stimuli in log units (0.22). When positive or negative responses were still observed with the 3.65 or 5.16 filament, values of 0.3 or 18.6 g were assigned, respectively, by assuming a value of 0.5 for k.
Statistical analysis
Results are presented as means±SEMs and analyzed using the SigmaStat program. Statistical analyses were done using two-way repeated-measures analysis of variances with two-repeated factors followed by Tukey post hoc test for the experiment of Latin square design or two-way repeated-measures analysis of variances with one repeated time factor followed by Tukey post hoc tests. In all cases, p<0.05 was considered significant.
RESULTS
SEP responses
SEP study, a measure of sensory nerve excitability, was extensively used in the diagnosis of neuropathies. Beginning from week 3, latency significantly started to increase in control groups (rolipram 16.76±1.27, ethanol 21.10±1.75, vehicle 21.19±1.85) ( p<0.05) ( Fig. 3). In rolipram-treated rats, the shorter latency was sustained until week 8, the final week of the study (rolipram 17.50±1.77, ethanol 26.30±2.55, vehicle 25.80±3.17) ( p<0.05) ( Fig. 3).
Mechanical threshold
The baseline mechanical thresholds of all rats before ethanol application was 17.9 g which was the maximal cutoff point. Ethanol decreased the mechanical threshold of both hind paws. Further, the mechanical threshold of both hind paws did not significantly differ from each other. Thus, we used the left hind paw as the site to measure mechanical threshold. When ethanol (10 g/kg) was administrated on day 0 to week 8, there was a significantly greater decrease in nociceptive threshold, beginning from week 3 (rolipram 18.56±1.943, ethanol 4.370±1.631, vehicle 4.375±1.663) ( p<0.05) ( Fig. 2). The statistical difference was sustained until week 8, the final week of the study (rolipram 16.60±1.53, ethanol 1.23±0.45, vehicle 1.38±0.55) ( p<0.05) ( Fig. 2).
DISCUSSION
The neurologic effects of ethanol consumption are complex, affecting on both the central and peripheral nervous system 26,32,35). In the peripheral nervous system, it produces a small-fiber dying back painful neuropathy 2,6). Over time, pain far outweighs analgesia, producing a neuropathic syndrome with symptoms that have been described as "like tearing flesh off the bones" 3). In recent years, ethanol consumption in Western industrialized countries has increased with an associated rise in the rates of alcohol-related health problems 8,11). Thus, understanding the basis of clinical expression of ethanol-induced neuropathy is an issue of growing importance, and adequate treatment of this symptom may require therapeutic strategies. Rolipram is a selective inhibitor of cAMP specific phosphordiesterase. PDE4 inhibitors have been reported to control epigenetic regulation and reduce both proinflammatory cytokine levels, including TNF-α and IL-1β 1,25), molecules involved in free radical production and oxidative stress such as iNOS and COX-2, as well as immune cell infiltration into the nervous system 28). Free radicals are derivatives of molecular oxygen and nitrogen and consist of superoxide, hydroxyl radical, hydrogen peroxide, and peroxynitrite 17). These molecules are ubiquitously present in the body and participate in many normal cellular processes, including ion transport, transcription, neurotransmission, and neuromodulation 17). Sources of free radicals are both mitochondrial oxidative metabolism which produces adenosine triphosphate and several enzymes such as xanthine oxidase, phospholipase A2, cytochrome P450, monoamine oxidase, and tyrosine hydroxylase. They are normally removed by antioxidant systems including superoxide dismutase, catalase, glutathione, glutathione peroxidase, ascorbate, and α-tocopherol. Thus, free radical levels are precisely controlled by antioxidant systems. However, in pathologic conditions, levels of free radicals may rise due to the increased production or decreased antioxidants level 9,10). Once a lesion develops in the central nervous system, two proinflammatory cytokines, TNF-α and IL-1β, are released. Numerous studies have documented rapid increases in TNF-α and IL-1β levels after nervous system injury such as traumatic brain injury and spinal cord injury occurs 15,33). IL-1β synergistically acts with TNF-α to induce nerve cell death. These proinflammatory cytokines stimulate inflammatory cells to release damaging reactive oxygen and nitrogen species, raise glutamate levels to excitotoxic levels, impair the ability of glia cells to buffer extracellular potassium, compromise the nervous system and attract inflammatory cells into the nervous system 13,14,20,29,30). Once initiated, the inflammatory cascade becomes a toxic positive-feedback loop, further exacerbating nervous system pathology. Several studies have demonstrated that restoration of cAMP levels improve outcome in models of nervous system injury. Rolipram which was used to inhibit the degradation of cAMP promoted axon sparing and improved functional outcomes 24). The effects of cAMP are short-lived because they are rapidly degraded by PDEs 18). Among ten PDE classes, two isoforms are highly selective for degrading cAMPs in the nervous system. Our present study determined for the first time that ethanol-induced allodynia was significantly restored in the rolipram-treated rats compared with control groups consumed ethanol and received vehicle injection ( Fig. 2, 3). Despite the impressive results of rolipram found in this study, cautious interpretation of study findings is warranted because of some limitation, such as study design without measurement of long-term effects and optimal dose of rolipram. Further study is needed to identify pathophysiology using cytokine analysis, ROS measurement and nerve biopsy.
CONCLUSION
This study shows that rolipram, a PDE4 inhibitor known to control epigenetic regulation and reduce both proinflammatory cytokine levels and molecules involved in free radical production and oxidative stress, ameliorated mechanical behavior as measured by mechanical allodynia and SEP in ethanol-induced neuropathy in rats.
Acknowledgements
The authors would like to thank Hee-Kee Kim, Associate Scientist, Dept. of Anesthesiology, University of Miami Miller school of Medicine, for his excellent behavioral and pharmaceutical advice. This work was supported by a research grant from Yonsei University Wonju College of Medicine, Institute of Occupational & Environmental Medicine.
References
1. Atkins CM, Oliva AA Jr, Alonso OF, Pearse DD, Bramlett HM, Dietrich WD : Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol 2007, 208 : 145-158,    2. Bosch EP, Pelham RW, Rasool CG, Chatterjee A, Lash RW, Brown L, et al : Animal models of alcoholic neuropathy : morphologic, electrophysiologic, and biochemical findings. Muscle Nerve 1979, 2 : 133-144,   3. Brain WR, Walton JN : Disorders of peripheral nerves : alcoholoc polyneuritis : Brain's deseases of the nervous system. 1969, 7th ed. London : Oxford, pp817-819
4. Cogswell JP, Godlevski MM, Wisely GB, Clay WC, Leesnitzer LM, Ways JP, et al : NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J Immunol 1994, 153 : 712-723,  5. Corsetti G, Rezzani R, Rodella L, Bianchi R : Ultrastructural study of the alterations in spinal ganglion cells of rats chronically fed on ethanol. Ultrastruct Pathol 1998, 22 : 309-319,   6. Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, et al : Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci 2000, 20 : 8614-8619,   7. Dixon WJ : Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980, 20 : 441-462,   8. Fillmore KM : Women's drinking across the adult life course as compared to men's. Br J Addict 1987, 82 : 801-811,   9. Floyd RA : Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med 1999, 222 : 236-245,   10. Floyd RA, Hensley K : Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging 2002, 23 : 795-807,   11. Gomberg ES : Women and alcohol : use and abuse. J Nerv Ment Dis 1993, 181 : 211-219,   12. Hou S, Guan H, Ricciardi RP : Phosphorylation of serine 337 of NF-kappaB p50 is critical for DNA binding. J Biol Chem 2003, 278 : 45994-45998,   13. Hu S, Peterson PK, Chao CC : Cytokine-mediated neuronal apoptosis. Neurochem Int 1997, 30 : 427-431,   14. Keeling KL, Hicks RR, Mahesh J, Billings BB, Kotwal GJ : Local neutrophil influx following lateral fluid-percussion brain injury in rats is associated with accumulation of complement activation fragments of the third component (C3) of the complement system. J Neuroimmunol 2000, 105 : 20-30,   15. Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD : Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats : importance of injury severity and brain temperature. Neurosurgery 2002, 51 : 195-203; discussion 2003,   16. Koike H, Iijima M, Sugiura M, Mori K, Hattori N, Ito H, et al : Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann Neurol 2003, 54 : 19-29,   17. Lander HM : An essential role for free radicals and derived species in signal transduction. FASEB J 1997, 11 : 118-124,  18. Manganiello VC, Murata T, Taira M, Belfrage P, Degerman E : Diversity in cyclic nucleotide phosphodiesterase isoenzyme families. Arch Biochem Biophys 1995, 322 : 1-13,   19. Mayr B, Montminy M : Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2001, 2 : 599-609,   20. Meda L, Cassatella MA, Szendrei GI, Otvos L Jr, Baron P, Villalba M, et al : Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 1995, 374 : 647-650,   21. Monforte R, Estruch R, Valls-Solé J, Nicolás J, Villalta J, Urbano-Marquez A : Autonomic and peripheral neuropathies in patients with chronic alcoholism. A dose-related toxic effect of alcohol. Arch Neurol 1995, 52 : 45-51,   22. Naik AK, Tandan SK, Dudhgaonkar SP, Jadhav SH, Kataria M, Prakash VR, et al : Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur J Pain 2006, 10 : 573-579,   23. Narita M, Miyoshi K, Narita M, Suzuki T : Involvement of microglia in the ethanol-induced neuropathic pain-like state in the rat. Neurosci Lett 2007, 414 : 21-25,   24. Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT : The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A 2004, 101 : 8786-8790,    25. Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, et al : cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med 2004, 10 : 610-616,   26. Pentney RJ, Quackenbush LJ : Dendritic hypertrophy in Purkinje neurons of old Fischer 344 rats after long-term ethanol treatment. Alcohol Clin Exp Res 1990, 14 : 878-886,   27. Schaal SM, Golshani R, Ghosh M, Lovera L, Lopez M, Patel M, et al : Targeting phosphodiesterase-4 after spinal cord injury using pharmacological and molecular approches. J Neurotrauma 2008, 25 : 297,
28. Sharma SS, Sayyed SG : Effects of trolox on nerve dysfunction, thermal hyperalgesia and oxidative stress in experimental diabetic neuropathy. Clin Exp Pharmacol Physiol 2006, 33 : 1022-1028,   29. Soares HD, Hicks RR, Smith D, McIntosh TK : Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci 1995, 15 : 8223-8233,  30. Tanaka M, Sotomatsu A, Yoshida T, Hirai S, Nishida A : Detection of superoxide production by activated microglia using a sensitive and specific chemiluminescence assay and microglia-mediated PC12h cell death. J Neurochem 1994, 63 : 266-270,   31. Verghese MW, McConnell RT, Strickland AB, Gooding RC, Stimpson SA, Yarnall DP, et al : Differential regulation of human monocyte-derived TNF alpha and IL-1 beta by type IV cAMP-phosphodiesterase (cAMP-PDE) inhibitors. J Pharmacol Exp Ther 1995, 272 : 1313-1320,  32. Victor M : Edited by Dyke PJ, Thomas PK, Lambert EH : Polyneuropathy due to nutritional deficiency and alcoholism : In: Peripheral neuropathy. 1975, Philadelphia : WB Saunders Co, pp1030-1066
33. Vitarbo EA, Chatzipanteli K, Kinoshita K, Truettner JS, Alonso OF, Dietrich WD : Tumor necrosis factor alpha expression and protein levels after fluid percussion injury in rats : the effect of injury severity and brain temperature. Neurosurgery 2004, 55 : 416-424; discussion 424-425,   34. Yerdelen D, Koc F, Uysal H : Strength-duration properties of sensory and motor axons in alcoholic polyneuropathy. Neurol Res 2008, 30 : 746-750,   35. Zou J, Rabin RA, Pentney RJ : Ethanol enhances neurite outgrowth in primary cultures of rat cerebellar macroneurons. Brain Res Dev Brain Res 1993, 72 : 75-84,  
Fig. 1
A: A schematic illustration of the rat skull with electrode placement for SEP study. B: SEP signal at different experimental stages in the ethanol induced neuropathy control group. SEP: somatosensory evoked potential. 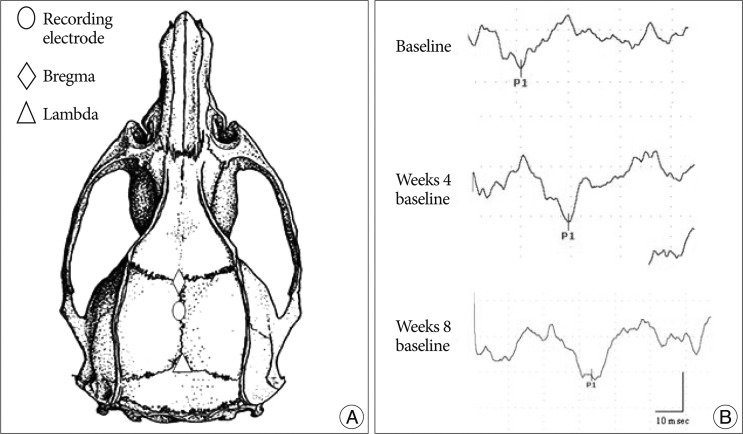
Fig. 2
The time courses of mechanical threshold in ethanol-induced neuropathy. Rolipram was intraperitoneally injected once a day. The vehicle group received equal volume of normal saline. Note that ethanol significantly decreased mechanical threshold for the first 3 weeks. Rolipram significantly ameliorated mechanical threshold decrease compared with ethanol diet and ethanol diet with vehicle injection groups (p<0.05). In this figure data are plotted as mean±standard error of the mean. Asterisks indicate values significantly different from those of ethanol diet alone and of vehicle group by using a two-way repeated measures analysis of variance with repeated time factor, followed by Tukey post hoc test. 
Fig. 3
The time courses of SEP latency in ethanol-induced neuropathy. Rolipram was intraperitoneally injected once a day. The vehicle group received equal volume of normal saline. Note that ethanol significantly increased the latency of SEP from the first 3 weeks. Rolipram significantly improved increased latencies compared with ethanol diet and ethanol diet with vehicle injection groups (p<0.05). In this figure data are plotted as mean±standard error of the mean. Asterisks indicate values significantly different from those of ethanol diet alone and of vehicle group by using a two-way repeated measures analysis of variance with repeated time factor, followed by Tukey post hoc test. EP: somatosensory evoked potential. 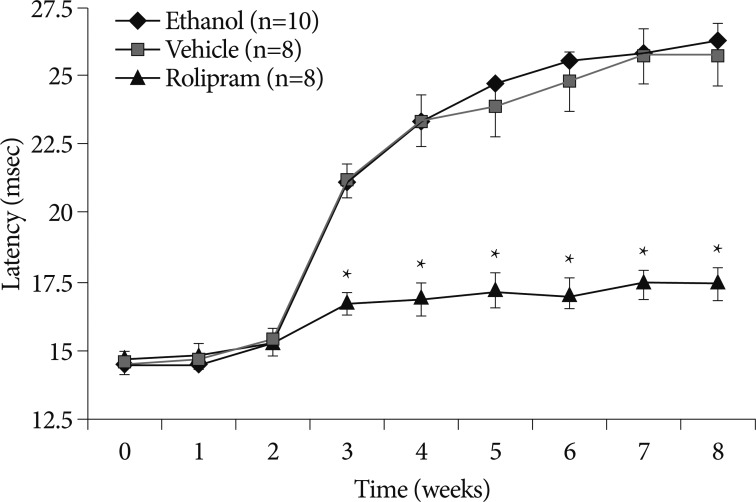
|
|


















