INTRODUCTION
Decompressive craniectomy is a critical treatment modality for the control of highly increased intracranial pressure10,18,21). Nonetheless, it can lead to large cranial defect that requires subsequent cranioplasty for neural tissue protection, successful cosmetic outcome and improvement of cerebral function4,20). An autogenous bone flap obtained at the time of the craniectomy can be used in subsequent cranioplasty because it is viable and fits well into the cranial defect. However, in many clinical situations, autogenous bone is unavailable because of bone resorption preventing good cosmetic outcome and cerebral protection14).
Recently, alloplastic materials including polymethyl-methacrylate (PMMA), hydroxyapatite, and metallic mesh have been suggested as intra-operative malleable substitutes. These materials, however, require complex processes, such as intraoperative mixing for the preparation, adaptation, and contouring of the implant for the defect, which result in increased surgical time. In addition, the molding process may lead to poor cosmetic outcomes in patients with large or complicated defects. Furthermore, the contouring of implant by direct contact to the dura can develop exothermic reactions or the release of toxic monomers intraoperatively. It results in focal tissue damage, which implicate local and systemic reactions9,19).
In order to overcome the shortcomings of intraoperative molding, many techniques have been introduced for the prefabrication of customized cranioplastic implants using medical imaing and three-dimensional (3D) biomodeling1,3,5,6,8,11,12,17,19,22). The prefabrication of alloplastic implants that are tailored to individual patients' defects not only improves aesthetic outcomes, but also decreases surgical time, blood loss, and the risk of infection16). In this study, the authors demonstrate a simple and cost-effective cranioplasty method using individualized mold prefabrication with a 3D printer and PMMA casting.
MATERIALS AND METHODS
Patient population
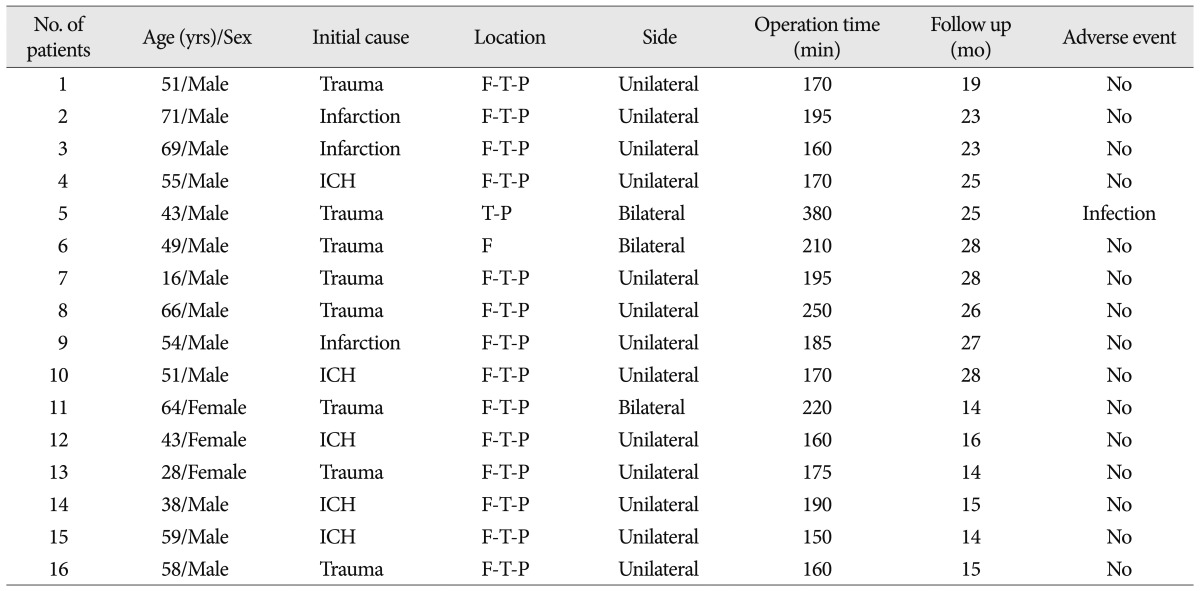
Between November 2009 and April 2011, 16 patients with large skull defects (>100 cm2) underwent cranioplasty with individualized prefabricated molds created by the 3D model and PMMA casting method at our institution. There were twelve male and four female. The median patient age at the time of cranioplasty was 52.5 years (range, 16 to 71 years). The cranial defects were caused by head trauma in eight cases (50%), cerebral infarction in three cases (19%), and intracerebral hemorrhage in five cases (31%). Thirteen patients had a unilateral cranial defect and three patients had a bilateral defect. Most of the cranial defects were located in the fronto-temporo-parietal region (88%) (Table 1).
Preparation of mold
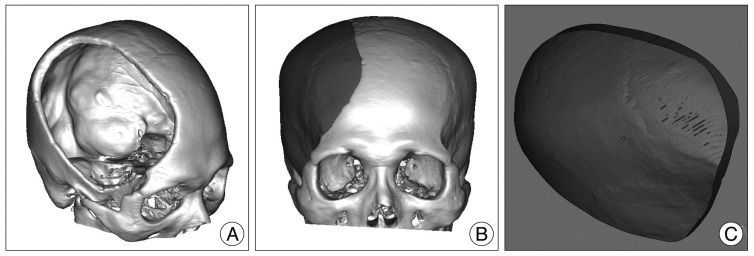
For unilateral cranial defect, Digital Imaging and Communications in Medicine (DICOM) data were acquired with the spiral volumetric technique during preoperative axial 1-mm spiral computed tomography (CT) scans (Philips Brilliance 64 Slice CT, Philips Healthcare, Best, The Netherlands). Subsequently, the DICOM data were processed and converted to 3D images with MIMICS 13.1 Software (Interactive Medical Image Control System; Materialise Inc., Leuven, Belgium) (Fig. 1A). The image of the implant was generated by a digital subtraction mirror-imaging process whereby the normal side of the cranium was used as a model (Fig. 1B, C). For bilateral cranial defects, preoperative spiral CT scan data (5- to 10-mm slice thickness) were obtained before the decompressive craniectomy (Fig. 2A). The stair-like surface of the 3D-implant model was processed with a smoothing technique by the MIMICS 13.1 Software (Materialise Inc.) (Fig. 2B). The pre- and postcraniectomy CT images were merged, and the 3D implant images were then cropped (Fig. 2C, D).
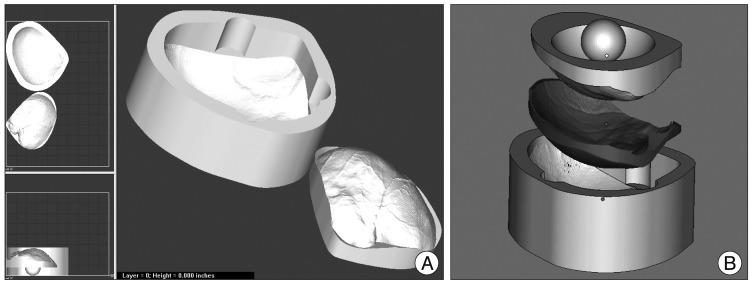
The 3D modeling of the mold was processed with Magics 15 software (Rapid Prototyping Software; Materialise Inc.) following the imaging of the required implant (Fig. 3). Prefabrication of the mold was performed by powder depositional modeling with the use of a Spectrum Z 510 3D Printer (Z Corporation, Burlington, MA, USA) (Fig. 4A, B). The prefabrication process took an average of 6 hours, and the mold was then sterilized by autoclave.
Surgical technique
All patients underwent cranioplasty under general anesthesia. After aseptic draping, a skin incision was made along a scar from a previous operation. Scalp tissue was retracted carefully not to penetrate the dura mater. The PMMA implant was constructed using the prefabricated mold during the dissection procedure. Due to the unknown biocompatibility of the prefabricated materials, the molds were wrapped with two layers of sterilized plastic. In order to prevent adhesion between the implant and the plastic, the surface of the plastic was moisturized with saline. The volume of PMMA was determined from the volume of the implant, which had been calculated preoperatively with the MIMICS 13.1 Software (Materialise Inc.). The PMMA resin was prepared by mixing polymer powder with a liquid monomer. In the liquid state, the PMMA resin was poured into the prefabricated molds and the molds were compressed to each other. The mold and plastic were separated from the PMMA implant after hardening (Fig. 4C). The implant required minor trimming around the margins in order to achieve an exact fit into the defective region. The PMMA implant was fixed to the defective region with titanium plates and self-tapping screws of 4 mm in length.
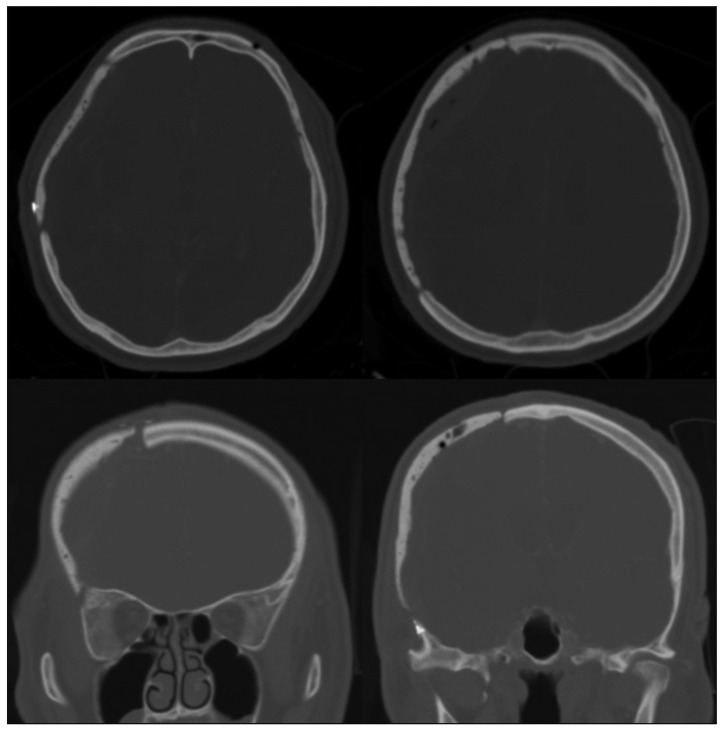
Plane X-rays of the skull and a CT scan were conducted immediately after surgery. A 3D reconstruction CT was performed one month later.
RESULTS
The median operation time was 184.36┬▒26.07 minutes (range, 150-380 minutes). The median follow-up period was 23 months (range, 14-28 months). Post-operative CT scans showed excellent restoration of the bony symmetry in all cases (Fig. 5). One patient who had an open wound defect developed a postoperative infection, which resulted in implant removal at 6 weeks. A representative case of a unilateral cranial defect and a case of bilateral cranial defects are described in this paper.
Case 2
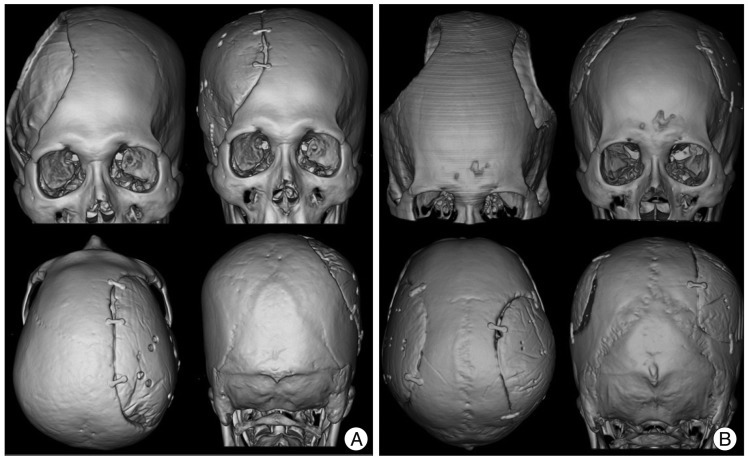
A 71-year-old woman underwent cranioplasty with individualized PMMA casting for the reconstruction of a large right fronto-temporo-parietal cranial defect. She had undergone a decompressive craniectomy for severe brain swelling that had been caused by a middle and posterior cerebral artery infarction 6 months before. Using the mirror-imaging process, an image of the cranial defect was generated, and the mold was prefabricated with the 3D printer. Intraoperatively, the PMMA implant was created with the mold and then fit into the defect. The post-operative 3D CT images showed excellent symmetric morphology (Fig. 6A).
Case 6
A 49-year-old man underwent a bilateral decompressive craniectomy due to traumatic brain swelling. A cranioplasty was performed three months later. By using pre- and postcraniectomy CT data, we applied the merge technique to obtain the precraniectomy cranial contour. A PMMA implant was created intraoperatively with the mold. The postoperative 3D CT images showed good restoration of the cranial deformity (Fig. 6B).
DISCUSSION
An intra-operative molding process is required in traditional cranioplasties using allografts, although it causes poor cosmetic result, increased operation time and tissue injury. In order to overcome these shortcomings, many techniques have been used for the prefabrication of customized cranioplastic implants1,3,5,6,8,11,12,16,19,22,23). However, there are some drawbacks for customized models including manufacturing time, biocompatibility, and cost. In this study, we developed a computer-generated model, which can be applied a prefabricated mold to the cranial defect. By using the PMMA casting method, we obtained a cranial implant that fit well into the anatomic defect. Our model may have beneficial effects on the clinical application for the reconstruction of cranial defects.
Craniofacial biomodels can be fabricated by conventional milling techniques, laser stereolithography, and 3D printing technology5,8,11,16,19,23). In 1990, the construction of biomodels using 4-axis computer numerical control milling techniques was first reported. These biomodels were then used as templates for implants or as a master tool for making molds for the fabrication of implant15). However, a major disadvantage of the conventional milling technique was that it was not able to accurately represent the complex anatomy or the inner structure of the craniofacial bones5). In the late 1990s, modified cranioplasty techniques were introduced to the reconstruction of complex or extensive cranial defects, consisting of 3D stereolithography and template modeling5,7). Laser stereolithography is accurate to less than 1 mm3, which is an improvement compared to conventional milling techniques. In addition, this technique demonstrated excellent results for the replication of complex geometric shapes5). In customized cranioplasty using stereolithography, the defect-bearing biomodel is utilized to mold a master implant following the creation of an impression mold of the cavity. Subsequently, a carbon-fiber reinforced polymer or acrylic implant is shaped by the mold5,19,23). However, the master implant must be constructed by hand into the defect in this model. In addition, the biomodel manufacturing time for stereolithography is about twice as long as that of 3D printing technology.
Recently, the development of 3D printing technology has introduced 3D medical models to generate an exact copy of various patients' skulls and facial bone structures8,16,17). It allows prefabricated copy models to simulate preoperative or intraoperative procedures3). In the present study, we applied 3D printing technology for the prefabrication of a mold which can create an implant for the reconstruction of the cranial defect. The image of the implant was generated by a digital subtraction mirror-imaging process whereby the normal side of the cranium was used as a model. We developed a 3D model by using high-resolution (1-mm cuts) spiral CT scans. The routine CT scans performed before the craniectomy were usually 5- to 10-mm thick. Consequently, the prosthesis had a stair-like surface because the 3D model was manufactured using the relatively thick spiral CT data. Therefore, a routine CT image may not be suitable for the creation of a precise model. However, bilateral decompressive craniectomy is sometimes performed in various clinical situations. It cannot develop precise model because there is no normal side of the cranium to use as a model. In order to overcome this problem, we used a precraniectomy routine CT image and modified it with a 3D model image program. We applied this model to manufacture bilateral PMMA casting in two cases. It can also be useful by minor trimming around the margins as shown in Fig. 6B, although bilateral PMMA casting doesn't fit well compared to unilateral one. It may be a first time report to develop customized cranioplasty implant in bilateral cranial defect.
By using our 3D model during the cranioplasty, the operation time may be reduced. In this study, the median operation time was 184.36┬▒26.07 min, whereas, in our previous report, it was 285┬▒128 min for cranioplasties using non-customized PMMA implants13). The reason is that the preparation of the implant is performed during the exposure of the cranial defect. In addition, most of the PMMA implants used in this study required only minor trimming before being fit into the cranial defects. A postoperative infection was found in one case (6.2%), who had an open wound defect previously. Considering the infection rate, this model is comparable to other procedures2). There were no other complications for 23 months follow-up period.
PMMA is one of the most biocompatible alloplastic materials currently available, and its use has long been established for cranioplasty. It allows for various forms of intra-operative molding or prefabrication. In addition, it has a relatively low cost, strong consistency and easy casting. However, PMMA induces an exothermic and potentially toxic nature of polymerization when it is conducted in situ. Polymerization can cause damage to sensitive dural and subdural structures and release monomers into the patient's blood circulation5,9,16). Therefore, a prefabricated implant has the advantage to prevent these potential complications of PMMA, as like our model.
Individualized prefabrication of the mold and PMMA casting is an easier and more cost-effective method than the previously customized cranial prostheses3,5,6,8,11,16,19). In the present study, we performed the cranioplasty in two steps : the prefabrication of the mold and the casting of the PMMA implant. It takes 1 and 6 hours to generate 3D images for the molding and prefabrication process, respectively. In addition, the cost of manufacturing a prefabricated mold is $450 USD approximately.
There are some considerations for our customized cranioplasty method in case of complex cranial defects. In cases of bilateral cranial defects and asymmetrical skulls, the mirror-image process cannot be used. Although we used precraniotomy routine CT scans (5- to 10-mm slices) to generate 3D images of the implant for the bilateral cranial defects, satisfactory results may sometimes not be obtained as readily as with unilateral cranial defects. In addition, the degree of skin contracture over large or complex cranial defects can cause a problem with skin closure5). Therefore, one must be careful to assess the degree of skin contracture required over large or complex cranial defects prior to implant design, although we did not have this problem.
CONCLUSION
In conclusion, cranioplasty using the individualized prefabrication of implants assures satisfactory aesthetic results, reduces surgical time, and decreases the potential complications associated with the use of PMMA. In addition, our customized cranioplasty model may have advantages with respect to cost, manufacturing time, and the simplicity of the procedure.