Kim and Kim: Clinical Comparison of 30-Day Mortalities and 6-Month Functional Recoveries after Spontaneous Intracerebral Hemorrhage in Patients with or without End-Stage Renal Disease
Abstract
Objective
The aim of this study was to determine 30-day mortality and 6-month functional recovery rates in spontaneous intracerebral hemorrhage (S-ICH) patients undergoing hemodialysis treatment for end-stage renal disease (ESRD), and to compare the outcomes of these patients and S-ICH patients without ESRD.
Methods
The medical records of 1943 S-ICH patients from January 2000 to December 2011 were retrospectively analyzed with focus on demographic, radiological, and laboratory characteristics.
Results
A total of 1558 supratentorial S-ICH patients were included in the present study and 102 (6.5%) were ESRD patients. The 30-day mortality of the S-ICH patients with ESRD was 53.9%, and 29.4% achieved good functional recovery at 6 months post-S-ICH. Multivariate analysis showed that age, Glasgow Coma Scale (GCS) score, pupillary abnormality, ventricular extension of hemorrhage, hemorrhagic volume, hematoma enlargement, anemia, and treatment modality were independently associated with 30-day mortality in S-ICH patients with ESRD (p<0.05), and that GCS score, volume of hemorrhage, conservative treatment, and shorter hemodialysis duration was independently associated with good functional recovery at 6 months post-S-ICH in patients with ESRD (p<0.05).
Conclusion
This retrospective study showed worse outcome after S-ICH in patients with ESRD than those without ESRD; 30-day mortality was four times higher and the functional recovery rate was significantly lower in S-ICH patients with ESRD than in S-ICH patients without ESRD.
Key Words: Spontaneous intracerebral hemorrhage ┬Ę Mortality ┬Ę Functional recovery ┬Ę Hemodialysis ┬Ę Predictor ┬Ę End-stage renal disease.
INTRODUCTION
Spontaneous intracerebral hemorrhage (S-ICH) accounts for 10% to 15% of all strokes and 78% to 88% of intracerebral hemorrhages 24). The 30-day mortality rate of S-ICH has been reported to range from 35% to 52% 24), and around half of all deaths occur during the first 2 days 3). In addition, S-ICH produces serious neurological sequelae that require long-term medical and social care 2,5). Several factors are known to predispose S-ICH, such as, hypertension, diabetes mellitus, dyslipidemia, and overweightedness 4,17,21,23,24,29). Recently, several reports have been issued on the prognostic value of hemodialysis in S-ICH patients with end-stage renal disease (ESRD). Cerebrovascular disease is the third most common cause of death in patients with ESRD 11,13). In these patients, the rate of S-ICH has been reported to range from 6.2 to 10.2 per 1000 individuals requiring chronic hemodialysis 14,16,19). Furthermore, the number of patients with ESRD requiring maintenance hemodialysis has been increasing 19), as is the number of S-ICH patients on chronic hemodialysis. Mortality among S-ICH patients with ESRD remains high and reportedly ranges from 50% to 90% 14,16). The outcome of the S-ICH patients on chronic hemodialysis treatment has been reported to be independently associated with high alcohol consumption, a short interval between onset and admission, impaired consciousness on admission, and a low fibrinogen level 6,7). Although some reports have described the risk factors and predictors of S-ICH 13,16,28), relatively few have addressed management strategies for improving patient prognosis in such cases 11,19). Management of S-ICH in hemodialysis patients is frequently complicated by several factors, such as hypotension during hemodialysis, active bleeding and coagulopathy that may be exacerbated by the systemic anticoagulant therapy used in hemodialysis, and dialysis disequilibrium syndrome attributing to edema of the central nervous system caused by a rapid osmolar shift, often resulting in increased peripheral edema and neurological deterioration 20). Although neurosurgeons occasionally encounter S-ICH attacks in patients with ESRD during hemodialysis in the clinical field, there have not been comprehensive studies show that the characteristics of this type of S-ICH compared with those of general S-ICH, nor been specific management guideline for these patients. Additionally, the factors associated with mortality and functional recoveries in S-ICH patients on hemodialysis for ESRD have not been widely studied. These limitations are also found in Korea.
In the present study, we retrospectively analyzed and compare the characteristics of S-ICH patients with or without hemodialysis for ESRD based on the data from a single institute. The primary study end-points were 30-day mortality and the 6-month functional recovery rate in both patients. In addition, we sought to identify predictors of 30-day mortality and 6-month functional recovery in patients with or without ESRD.
MATERIALS AND METHODS
Data collection
We retrospectively studied consecutive patients with a diagnosis of S-ICH admitted to the Stroke Unit at our single hospital (a university hospital serving a population of 1500000) between January 2000 and December 2011. Patients aged greater than 40 years were selected because of the likelihood of a secondary etiology in younger patients. In addition, we excluded patients with an infratentorial hemorrhage, because small changes in hemorrhage size or location are believed to have greater impacts on survival than supratentorial hemorrhages in these patients. Patient related data were independently extracted from a computerized database (PACS; Marosis m-view, Marotech Co., Seoul, Korea) by three clinical research coordinators using a structured form. To preserve patient confidentiality, patient identifiers were omitted from the collated data set. The inclusion criteria applied were a diagnosis of supratentorial S-ICH, confirmed by computerized tomography (CT) or magnetic resonance imaging, and admission to a stroke unit within 24 hours of symptom onset. As recommended by the Stroke Council of the American Heart Association 1,2), selected patients underwent conventional angiography to identify secondary causes. Patients with a hemorrhage secondary to head trauma, a ruptured cerebral aneurysm, an arteriovenous malformation, a tumor, bleeding diathesis, or a hemorrhagic infarction were excluded. This study was approved by the institutional review boards of our hospital (2012-SCMC-065-00).
Hemodialysis in ESRD patients
Hemodialysis was performed using Gambro AK 95® units (Gambro, Stockholm, Sweden) equipped with a Polyamix® dialysis membrane (Gambro) using Bicart® (Gambro) dialysis solution. Hemodialysis was performed three times a week at 4 hours/day at a mean blood flow rate of 150-200 mL/min. The anticoagulant (heparin) loading dose was 1000 IU and was intravenously infused, and the maximum maintenance dose was 200 IU per hour. In addition, nafamostat mesylate was intravenously infused at an hourly dosage of 35 mg without a loading dose.
Determination of prognostic variables
The baseline variables recorded were selected by reviewing the literature 21,25,29). Demographic variables included; age, gender, cigarette smoking and alcohol intake histories, body mass index (BMI), level of consciousness, pupillary abnormalities, limb weakness, and underlying disease. Smoking status was classified as never smoker, ex-smoker (a lifelong smoking history of Ōēź6 months), or current smoker (Ōēź10 cigarettes/day for Ōēź6 months), and alcohol intake was dichotomized as < or Ōēź46 g/day 3 days/week 12). BMI was calculated by dividing weight in kilograms by height in meters squared. Pupillary abnormalities at admission were classified as 0, 1, or 2 based on pupil reactivity scores 17). Levels of consciousness at admission were assessed using the Glasgow Coma Scale (GCS) 27); GCS scores of 3-8, 9-12, and 13-15 were defined as 'severely altered', 'moderately altered', or 'mildly altered or normal' conscious states, respectively. Blood pressure at admission was not viewed as a potential prognostic variable because it is often markedly elevated during the first 1 to 2 days after a severe stroke.
Radiologic and laboratory characteristics
CT variables, that is, side of hemorrhage, location of hemorrhage, volume of hematoma, lateral shift of cerebral midline structures, and ventricular extension of hemorrhage, were also included in the analysis. Hematoma volume was estimated from axial CT scans using the formula A├ŚB├ŚC/2, where A is greatest diameter on the largest hemorrhage slice, B is maximal diameter perpendicular to this, and C is vertical hematoma depth 18). Hematoma locations were defined based on sites of origin, such as, basal ganglia, thalamus, lobal, or ventricle alone. Ventricular extension of hemorrhage included a pure intraventricular hemorrhage and secondary spread of cerebral parenchymal hemorrhage into ventricular space. A CT scan was performed whenever neurological status changed and 48 hours after the initial CT scan if neurological status was stable. Hematoma enlargement was defined as an increase in hematoma volume of Ōēź25% versus initial CT volume. Laboratory parameters obtained at admission were dichotomized during the analysis; these were hemoglobin, leukocyte count, platelet count, C-reactive protein (CRP) level, blood urea nitrogen (BUN), creatine, prothrombin time and activated partial prothrombin time. International Normalized Ratio (INR), plasma total cholesterol level, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood plasma glucose, and hemoglobin A1c.
Treatment modalities
Patients were treated in accord with institutional treatment algorithms. All patients (medical and surgical) were cared for in a neurosurgical intensive care unit until considered stable enough to move to a general unit. Treatment was administered according to current practices, but was not rigidly regimented as primary attending neurosurgeons were allowed to exercise medical judgment.
Surgical intervention was undertaken in selected patients exhibiting clinical deterioration and a moderate or larger amount of lobar hemorrhage, and in patients with basal ganglionic or thalamic hemorrhage with a hematoma volume of >30 mL, in patients with an expanding hematoma, and in patients with evidence of progressive neurological deterioration. Surgical approaches were individualized based on S-ICH location and size. The techniques used were craniotomy, craniectomy, CT-guided stereotactic hematoma evacuation, and/or extraventricular drainage. Patients with a deep-seated S-ICH usually underwent stereotactic evacuation when hematoma volume was from 30 to 60 mL by preoperative CT. Immediately after initial hematoma aspiration or evacuation, patients underwent repeat CT to check catheter placement. When repeat CT showed remnant hematoma, a urokinase (6000 U) injection was considered through the catheter to facilitate aspiration, and a CT scan was repeated 12 hours later. Patients with a hemorrhage of 10 to 30 mL and a severe neurological deficit (limb weakness) were also treated by stereotactic evacuation. On the other hand, when hematoma volume exceeded 60 mL, evacuation was performed via craniotomy or decompressive craniectomy. The attending neurosurgeon decided whether to implant a bone-flap (craniotomy or craniectomy) depending on the intraoperative presence of cerebral swelling after S-ICH removal. Conservative treatment mainly involved the management of hypertension and intracranial pressure. Blood pressure was controlled using several medications. Mean arterial pressure was maintained in the range 100 to 140 mm Hg using an antihypertensive (e.g., labetalol, amolodipine, or nicardipin). In addition, a hypertonic agent (e.g., glycerol or mannitol) was administered when CT indicated a mass effect or when clinical symptoms indicated an elevated intracranial pressure.
Assessments of functional outcomes
Patients were followed up for at least 6 months after S-ICH. Functional outcomes were assessed using the Glasgow Outcome Scale (GOS) in 6 months after ictus. GOS scores were defined as follows 15) : 1=dead, 2=vegetative state, 3=severe disability (able to follow commands, but unable to live independently), 4=moderate disability (able to live independently, but unable to return to work or school), 5=good recovery (able to return to work or school). For statistical purposes, patients were assigned to two outcome category groups, namely, a "good functional recovery" group (GOS score 4 to 5), members of which were functionally independent, or a "poor functional recovery" group (GOS score 1 to 3). Information on mortality and functional outcome was obtained from medical records, information obtained from family members was obtained by conducting telephone interviews, and information from patients was obtained by conducting direct examinations at our outpatient department after discharge.
Statistical analysis
Our primary aim was to estimate 30-day mortality and functional recovery at 6-month post-S-ICH in S-ICH patients on chronic hemodialysis for ESRD patients. We defined 30-day mortality as death within 30 days of a radiological diagnosis of S-ICH, and functional recovery was defined as described by GOS at 6 months after a radiological diagnosis of S-ICH.
SPSS version 12.0 (SPSS Institute, Inc., Chicago, IL, USA) was used for the analysis, and the Student's t test and a nonparametric test were used to analyze continuous and ordinal variables (e.g., mean levels of BUN and creatine), respectively. Discrete variables, such as, gender, smoking, chronic alcohol intake, ventricular extension of hemorrhage, and hydrocephalus and categorical variables, such as, age subgroup, BMI, GCS subgroup, pupillary abnormality, underlying conditions, and location of hemorrhage, were analyzed using Pearson's Žć2 test. Univariate analysis was used initially to identify relationships between potential prognostic factors and 30-day mortality and functional recovery at 6 months post-S-ICH using the Žć2 test. Subsequently, multivariate logistic regression analysis was used to identify variables independently associated with the above-mentioned outcomes. Kaplan-Meier survival analysis was used to determine cumulative mortalities. Statistical significance was accepted for p values of <0.05, and all tests were 2-tailed.
RESULTS
From January 2000 to December 2011, 1943 cases of S-ICH were diagnosed at the stroke units in our institute. Of these, 1558 supratentorial S-ICH patients were included in the present study; 1456 (93.5%) did not have ESRD, and 102 (6.5%) were ESRD patients on hemodialysis. Mean patient age was 66.4┬▒16.3 years (range 40 to 91 years), and 771 (49.5%) of the study subjects were male. Among of the 102 S-ICH patients with ESRD, mean age was 62.3┬▒18.6 years (ranged 37 to 86 years) and 55 (54.0%) were male. Mean duration of hemodialysis in these patients was 65.2 months (range from 1.0 month to 241.2 months). Causes of ESRD were hypertension in 34 (33.3%), diabetes mellitus in 49 (48.0%), polycystic kidney disease in 10 (9.8%), and chronic glomerulonephritis in 9 (8.8%).
The clinical characteristics of S-ICH patients with or without ESRD
No significant intergroup differences were observed between demographic characteristics, that is, age, gender, chronic alcohol intake, body mass index, or new limb weakness. However, ESRD patients had a lower mean GCS score ( p=0.028), and more ESRD patients had nonreactive pupils ( p=0.040) and underlying diseases ( p=0.013) ( Table 1). In terms of radiologic characteristics, no significant intergroup difference was found for sidedness or hemorrhagic volume, midline shift, location of hemorrhage, or hydrocephalus. However, ventricular extension of hemorrhages ( p=0.010), and hematoma enlargement within 48 hours were more prevalent in the ESRD patients ( Table 2). In terms of laboratory findings, no significant intergroup difference was found for leukocytosis (Ōēź10├Ś10 3/┬ĄL), hepatopathy (AST or ALT >40 IU/L), or elevated hemoglobin A1c (Ōēź6.0%). However, anemia (<11.7 g/dL in male, <10.7 g/dL in female) ( p<0.001), thrombocytopenia (<150├Ś10 3/┬ĄL) ( p=0.032), elevated INR (>1.2) ( p=0.004), elevated CRP (>5.0 mg/L) ( p=0.002), elevated blood plasma glucose (>126 mg/dL) ( p<0.001), elevated BUN (>24 mg/dL) ( p<0.001), elevated serum creatine (>1.4 mg/dL) ( p<0.001), and abnormal findings of urine analysis ( p<0.001) were more prevalent in S-ICH patients with ESRD ( Table 3). In these patients, mean BUN was 54.2 mg/dL (range from 10.7 mg/dL to 145.9 mg/dL) and mean serum creatine was 7.7 mg/dL (range from 2.9 mg/dL to 27.7 mg/dL). Urine output was nil in 43 S-ICH patients with ESRD (42.2%); proteinuria was present in 49 (48.0%), glucosuria in 24 (23.5%), and hematuria in 17 (16.7%) ( Table 3). Regarding treatment modalities, 1005 (64.5%) patients were treated conservatively and 553 (35.5%) were treated surgically. No significant intergroup difference was found in terms of treatment modality type ( p=0.667). Furthermore, of the 553 S-ICH patients that underwent surgical treatment, no significant difference was found between S-ICH patients with or without ESRD in terms of surgical techniques ( p=0.605) or time to surgery ( p=0.311) ( Table 4).
Clinical outcomes
For all study subjects, 30-day, 3-month, and 6-month mortalities were 16.4%, 25.7%, and 27.3%, respectively. Overall, 256 patients died within 30 days, 401 patients within 3 months, and 425 patients within 6 months of S-ICH. A significant and meaningful intergroup difference was found between 30-day ( p<0.001), 3-month ( p<0.001), and 6-month ( p<0.001) mortalities ( Table 5). In terms of functional recovery, at 30 days after S-ICH, GOS scores were similar ( p=0.545), but at 3 months ( p=0.046) and 6 months after S-ICH GOS scores were significantly different ( p=0.013) ( Table 5).
Prognostic factors of 30-day mortality
In patients without ESRD, univariate analysis showed that the following variables were significantly associated with death within 30 days of S-ICH; GCS score, pupillary abnormality, underlying disease, ventricular extension of hemorrhage, hemorrhagic volume, thrombocytopenia, and treatment modality. In S-ICH patients with ESRD in addition to these factors, age, anemia, and C-reactive protein were also found to be associated with death within 30 days of S-ICH.
Multivariate logistic regression analysis was performed on variables found to be significant by univariate analysis ( Table 6). In the S-ICH patients without ESRD, multivariate analysis showed that GCS score (3 to 8 vs. 9 to 12; p=0.038, and 3 to 8 vs. 13 to 15; p<0.001), pupillary abnormality (0 reactive vs. 2 reactive; p<0.001), treatment modality (surgery vs. conservative treatment; p=0.006, and craniotomy vs. other surgical modalities; p<0.001), underlying disease (ischemic heart disease vs. other disease; p=0.034, and atrial fibrillation vs. other disease; p=0.018) were independently associated with 30-day mortality. In the S-ICH patients with ESRD, multivariate analysis showed that age (Ōēź70 years vs. 40 to 54 years; p=0.019), GCS score (3 to 8 vs. 13 to 15; p<0.001, and 9 to 12 vs. 13 to 15; p=0.014), pupillary abnormality (0 reactive vs. 1 reactive; p=0.032, and 0 reactive vs. 2 reactive; p<0.001), ventricular extension of hemorrhage (present vs. absent; p=0.017), hemorrhagic volume (Ōēź60 mL vs. <30 mL; p=0.002, and 30-59 mL vs. <30 mL; p=0.029), anemia (present vs. absent; p=0.008), and treatment modality (surgery vs. conservative treatment; p=0.034), were independently associated with high 30-day mortality. Associations between other variables and 30-day mortality by univariate analysis were not confirmed by multivariate analysis. In S-ICH patients with ESRD, duration of hemodialysis was not associated with 30-day mortality ( p=0.199). Actually, underlying disease, which was independently associated with high 30-day mortality in S-ICH patients without ESRD, was not associated with high 30-day mortality in S-ICH patients with ESRD. Conversely, several other variables, such as, ventricular extension of hemorrhage, hemorrhagic volume, and anemia, not associated with 30-day mortality in S-ICH patients without ESRD, were found to be significantly and independently associated with 30-day mortality in S-ICH with ESRD.
Prognostic factors of functional recovery at 6 months after S-ICH
Of the variables examined, the following were found to be significantly associated with functional recovery in S-ICH patients with and without ESRD by univariate analysis; age, GCS score, location of hemorrhage, hemorrhagic volume, and treatment modality. However, pupillary response, underlying disease, ventricular extension of hemorrhage, anemia, thrombocytopenia, and CRP, which were all found to be associated with 30-day mortality, were not found to be associated with functional recovery in either group by univariate analysis.
In patients without ESRD, multivariate logistic regression analysis showed that the following five variables were independently associated with functional recovery at 6 months after S-ICH : age (40 to 54 years vs. Ōēź70 years; p<0.001, and 40 to 54 years vs. 55 to 69 years; p=0.038), GCS score (9 to 12 vs. 3 to 8; p=0.009, 13 to 15 vs. 3 to 8; p<0.001, and 13 to 15 vs. 9 to 12; p=0.041), location of hemorrhage (thalamus vs. other regions; p=0.022, lobal vs. other regions; p=0.042, and ventricle only vs. other regions; p=0.008), volume of hemorrhage (<30 mL vs. Ōēź60 mL; p=0.001), and treatment modality (conservative treatment vs. surgery; p=0.039, other surgical modalities vs. craniotomy; p<0.001, and stereotactic aspiration vs. other surgical modalities; p=0.005).
In S-ICH patients with ESRD, multivariate logistic regression analysis showed that the following four variables were independently associated with functional recovery at 6 months after S-ICH; GCS score (9 to 12 vs. 3 to 8; p<0.001, and 13 to 15 vs. 3 to 8; p<0.001), hemorrhagic volume (<30 mL vs. Ōēź60 mL), treatment modality (conservative treatment vs. surgery; p<0.001), and duration of hemodialysis treatment (<50 months vs. Ōēź50 months; p=0.027). Although age and location of hemorrhage were associated with good functional recovery by univariate analysis in S-ICH patients with ESRD, multivariate analysis showed no such association. Thus, age and location of hemorrhage were associated with functional recovery in S-ICH patients without ESRD, but not in S-ICH patients with ESRD ( Table 7).
DISCUSSION
Of the estimated 67000 individuals who experienced S-ICH in the United States during 2002, only 20% were expected to be functionally independent 6 months later 3). Furthermore, unlike the declining mortality rates of subarachnoid hemorrhage and arteriovenous malformation, which have been attributed to improvements in surgical and critical care techniques, morbidity and mortality after S-ICH have remained substantially unchanged for decades 17). S-ICH is relatively common in the Far East 4,23), and in South Korea, cerebrovascular disease is the second most common cause of death, for example, in 2009, 90.1 deaths were recorded per 100000 population deaths 26). Several studies have reported the prognostic values of hemodialysis in S-ICH patients with ESRD. A 22-year (1980-2002) study performed Toyoda et al. 28) in 1740 ESRD patients, it was found that stroke occurred in 61 hemodialysis patients during the first 17 years and in 90 patients during the just in the last 5 years. Furthermore, it has been reported that the incidence of hemorrhagic stroke among hemodialysis patients is approximately twice that of ischemic stroke 16). Although technical improvements in hemodialysis continue to prolong the life-spans of these patients, the rate of treated ESRD continues to increase globally, and as a result, the numbers of ESRD patients that experience spontaneous ICH is set to increase. This retrospective study, which is the largest of its type undertaken in Korea, was performed to determine 30-day mortality and 6-month functional recovery after S-ICH in ESRD patients on chronic hemodialysis, and compare these results with those of the general S-ICH population. Several studies have examined mortalities in S-ICH patients on hemodialysis. In a prospective study, Huang et al. 10) found that overall 30-day mortality was 52.6% (20 deaths among 38 patients). In addition, several retrospective studies have reported overall mortalities ranging from 36.5% to 78.9% 8,9,16,20). Similarly, in the present study, 30-day mortality was 53.9%. Interestingly, Molshatzki et al. 20) reported that mortality is dependent on degree of renal function impairment. In this previous study, chronic kidney disease was categorized by estimated baseline glomerular filtration rate into moderate/severe impairment (<45), mild impairment (45-60), and no impairment (>60 mL/min/1.73 m 2). The authors reported 30-day mortalities of 41% for patients with no impairment, 30% for mild impairment, and 83% for moderate/severe impairment 20). In the present study, GCS score, pupillary response, and treatment modality were found to be independently associated with 30-day mortality in both of the study groups. However, several factors not related to 30-day mortality in non-ESRD patients (age Ōēź70, ventricular extension of hemorrhage, hemorrhagic volume Ōēź30 mL, anemia, and enlargement of hematoma within 48 hours) were associated with 30-day mortality in ESRD patients by multivariate analysis using a logistic regression model. Conversely, underlying diseases, such as, ischemic heart disease and atrial fibrillation, which were independently associated with 30-day mortality in non-ESRD patients, were associated with 30-day mortality in ESRD patients. Additionally, although hemorrhagic locations were associated with functional recovery in S-ICH patients without ESRD, they were not associated with functional recovery in S-ICH patients with ESRD.
Huang et al. 9) suggested that initial GCS score, age >80 years, an infratentorial location, hemorrhage volume, and ventricular extension of hemorrhage are associated with mortality rate in general S-ICH population, but that these associations do not hold in S-ICH patients with ESRD. As a result, these authors devised a scoring system based on a combination of initial GCS score, age, and initial systolic blood pressure (SBP), and stratified ESRD patients to determine their roles as predictors of mortality in S-ICH patients with ESRD. It was concluded that the three above-mentioned factors are optimal predictors of 30-day mortality for S-ICH patients with ESRD. Similarly, we found that initial GCS score and age were independent predictors of 30-day mortality in these patients. However, this previously study was limited by the exclusion of patients that underwent surgery or whose families requested DNR. The GCS is a standard neurological assessment tool is often used to predict cerebrovascular disease outcome 8). Like the majority of previous studies on predictive models, we classified patients using GCS scores of 3-8, 9-12, and 13-15. The GCS scores of S-ICH patients with ESRD were found to be lower than those of non-ESRD patients. Furthermore, we found that a lower GCS score was independently associated with high 30-day mortality rate and a low 6-month functional recovery rate by logistic regression analysis after adjustment. The 30-day mortality rates of S-ICH patients with ESRD were 71.4% for a GCS score of 3-8, 56.4% for a score of 9-12, and 14.3% for a score of 13-15. Hypertension may play an important role in the pathogenesis of cardiovascular and cerebrovascular morbidity and mortality (which is approximately 24% among patients with cerebrovascular diseases and 60-90% among hemodialysis patients in US) 22). Furthermore, a 'U' curve relationship between SBP and cerebrovascular mortality in hemodialysis patients has been shown 30). Huang et al. 9) compared two subgroups in their hemodialysis cohort of S-ICH patients in Taiwan during 1994-2004 with a SBP of 130-199 mm Hg vs. those with a SBP of <130 mm Hg or >200 mm Hg and found the latter was strongly related to 30-day mortality. In fact, we tried to reduce elevated blood pressure after admission using anti-hypertensives, such as, labetalol and nicardipine. The important point here is that concise information is required about the optimal control of blood pressure before a S-ICH attack, because the continuous control of blood pressure may play a critical role in the development of cerebrovascular disease 9). Actually, hematomas in patients undergoing hemodialysis are larger and more likely to increase in size after symptom onset due to an increased bleeding tendency and increase the risk of death. Moreover, outcomes are especially poor in patients that demonstrate hematoma enlargement after hospitalization 7). In terms of hematoma enlargement, Miyahara et al. 19) reported that all S-ICH patients exhibiting this condition died within 30 days of S-ICH, and suggested that an elevated INR predicts hematoma enlargement. Although we also found that hematoma enlargement was associated with high mortality in S-ICH patients with ESRD, elevated INR was not found to be an independent predictor of mortality in these patients. In the study by Miyahara et al., all patients received conservative treatment, but in the present study, some patients underwent surgical treatment, which might explain this discrepancy. Although the present study is the largest Korean study conducted to date on the clinical outcomes of S-ICH patients being treated by hemodialysis for ESRD, it has several limitations. First, its main limitation is the inherent bias introduced by its retrospective nature. We attempted to reduce this bias by collecting patient data from complete medical and radiological records and by recruiting patients treated using the same protocol. However, the evaluation was not complete to compare the clinical outcome (e.g., 30-day mortality and 6-month functional recovery) in both groups directly, because of basic differences in clinical characteristics between two groups. To overcome this limitation, prospective and randomized clinical trial must be essential. Second, although patients with ESRD have markedly advanced atherosclerosis of the cerebral and coronary arteries and frequently succumb to cardiovascular disease, the conditions of their hearts and cardiovascular systems are not routinely monitored because echocardiography is not routinely carried out in emergency rooms. Thus, we believe that appropriate cardiovascular factors could be important prognostic factors. Third, although it is known that chronic hypertension is a critical contributor to the development of cerebrovascular disease, we were unable to investigate the relation between hypertension and clinical outcome in S-ICH patients with ESRD due to lack of long-term data before S-ICH. Forth, detailed analysis of the relation between renal function and clinical outcome was not performed, and as was reported by Molshatzki et al. 20) severity of renal impairment may influence outcomes.
CONCLUSION
This retrospective study shows that 30-day mortality was four times higher, and that the functional recovery rate was significantly lower in S-ICH patients with ESRD than in S-ICH patients without ESRD. Demographically, S-ICH patients with ESRD had poorer GCS scores, poorer pupillary response, and more underlying disease than S-ICH patients without ESRD, and in terms of radiologic and laboratory findings, intraventricular hemorrhage, hematoma enlargement within 48 hours, anemia, and thrombocytopenia were more common in S-ICH patients with ESRD than in those without.
Based on our comparison of S-ICH patients with or without ESRD, this study shows age, ventricular extension of hemorrhage, hemorrhagic volume, anemia, and hematoma enlargement within 48 hours increases the risk of mortality, and that duration of hemodialysis treatment affects functional recovery adversely in S-ICH patients with ESRD. Accordingly, although we think that the effective management of these risk factors may improve the outcomes of ESRD patients on hemodialysis that experience S-ICH, a prospective randomized clinical trial is needed to confirm our results.
Acknowledgements
The authors would like to thank Yun Gyu Song, M.D. and Ha Young Lee, M.D. (Department of Radiology) for their review of neuroradiological images, Young Wook Kim, M.D. (Department of Biostatistics) for assistance with the statistical analysis, and three clinical research coordinators, Sa Eun Ahn, Hyun Jin Kim, and Kyoeng Ran Kim for collecting data.
References
1. Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al : Guidelines for the management of spontaneous intracerebral hemorrhage in adults : 2007 update : a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007, 38 : 2001-2023,   2. Broderick JP, Adams HP Jr, Barsan W, Feinberg W, Feldmann E, Grotta J, et al : Guidelines for the management of spontaneous intracerebral hemorrhage : a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1999, 30 : 905-915,   3. Counsell C, Boonyakarnkul S, Dennis M, Sandercock P, Bamford J, Burn J, et al : Primary intracerebral haemorrhage in the Oxfordshire Community Stroke Project, 2 : prognosis. Cerebrovasc Dis 1995, 5 : 26-34,  4. Epidemiology of cerebrovascular disease in Koreaa Collaborative Study, 1989-1990. Korean Neurological Association. J Korean Med Sci 1993, 8 : 281-289,    5. Evers SM, Ament AJ, Blaauw G : Economic evaluation in stroke research : a systematic review. Stroke 2000, 31 : 1046-1053,   6. Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R : Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke 1998, 29 : 1160-1166,   7. Fujii Y, Tanaka R, Takeuchi S, Koike T, Minakawa T, Sasaki O : Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg 1994, 80 : 51-57,   8. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC : The ICH score : a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001, 32 : 891-897,   9. Huang BR, Liao CC, Huang WH, Hsu YH, Hsu JC, Yen HC, et al : Prognostic factors of spontaneous intracerebral haemorrhage in haemodialysis patients and predictors of 30-day mortality. Intern Med J 2008, 38 : 568-574,   10. Huang BR, Lo YL, Chang CH, Chen TY : Testing the outcome score of spontaneous intracerebral haemorrhage in haemodialysis patients. Intern Med J 2009, 39 : 692-695,   11. Ikeda K, Tsuchimochi H, Takeno Y, Yasuda M, Fukushima T, Toyoda K : [Clinical analysis of the patients with hemodialysis associated with intracerebral hematoma]. No Shinkei Geka 2004, 32 : 1133-1137,  12. Ikehara S, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, et al : Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women : the Japan collaborative cohort study. Stroke 2008, 39 : 2936-2942,   13. Iseki K, Fukiyama K : Predictors of stroke in patients receiving chronic hemodialysis. Kidney Int 1996, 50 : 1672-1675,   14. Iseki K, Fukiyama K : Okawa Dialysis Study (OKIDS) GroupClinical demographics and long-term prognosis after stroke in patients on chronic haemodialysis. The Okinawa Dialysis Study (OKIDS) Group. Nephrol Dial Transplant 2000, 15 : 1808-1813,   15. Jennett B, Bond M : Assessment of outcome after severe brain damage. Lancet 1975, 1 : 480-484,  16. Kawamura M, Fijimoto S, Hisanaga S, Yamamoto Y, Eto T : Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis 1998, 31 : 991-996,   17. Kim KH, Kim HD, Kim YZ : Comparisons of 30-day mortalities and 90-day functional recoveries after first and recurrent primary intracerebral hemorrhage attacks : a multiple-institute retrospective study. World Neurosurg 2013, 79 : 489-498,   18. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al : The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996, 27 : 1304-1305,   19. Miyahara K, Murata H, Abe H : Predictors of intracranial hematoma enlargement in patients undergoing hemodialysis. Neurol Med Chir (Tokyo) 2007, 47 : 47-51; discussion 51-52,   20. Molshatzki N, Orion D, Tsabari R, Schwammenthal Y, Merzeliak O, Toashi M, et al : Chronic kidney disease in patients with acute intracerebral hemorrhage : association with large hematoma volume and poor outcome. Cerebrovasc Dis 2011, 31 : 271-277,   21. Nilsson OG, Lindgren A, Brandt L, S├żveland H : Prediction of death in patients with primary intracerebral hemorrhage : a prospective study of a defined population. J Neurosurg 2002, 97 : 531-536,   22. Ochiai H, Uezono S, Kawano H, Ikeda N, Kodama K, Akiyama H : Factors affecting outcome of intracerebral hemorrhage in patients undergoing chronic hemodialysis. Ren Fail 2010, 32 : 923-927,   23. Park HS, Kang MJ, Huh JT : Recent epidemiological trends of stroke. J Korean Neurosurg Soc 2008, 43 : 16-20,    24. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF : Spontaneous intracerebral hemorrhage. N Engl J Med 2001, 344 : 1450-1460,   25. Roquer J, Rodr├Łguez Campello A, Gomis M, Ois A, Puente V, Munteis E : Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol 2005, 252 : 412-416,   27. Teasdale G, Jennett B : Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2 : 81-84,  28. Toyoda K, Fujii K, Fujimi S, Kumai Y, Tsuchimochi H, Ibayashi S, et al : Stroke in patients on maintenance hemodialysis : a 22-year single-center study. Am J Kidney Dis 2005, 45 : 1058-1066,   29. Willmot M, Leonardi-Bee J, Bath PM : High blood pressure in acute stroke and subsequent outcome : a systematic review. Hypertension 2004, 43 : 18-24,   30. Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, et al : "U" curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 1998, 54 : 561-569,  
Table┬Ā1
Demographic characteristics of the patients with a spontaneous intracerebral hemorrhage 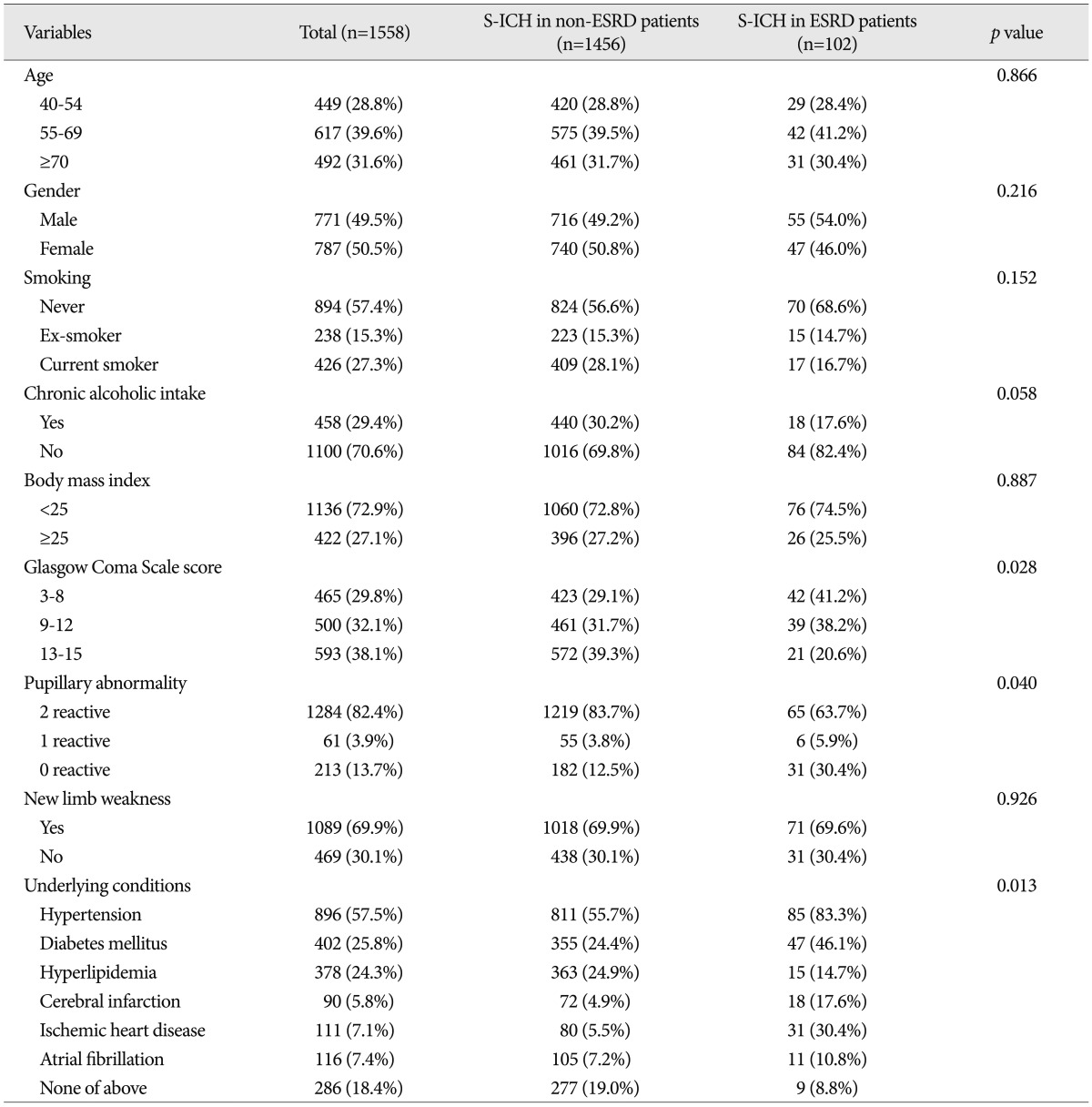
Table┬Ā2
Radiologic characteristics of patients with a spontaneous intracerebral hemorrhage 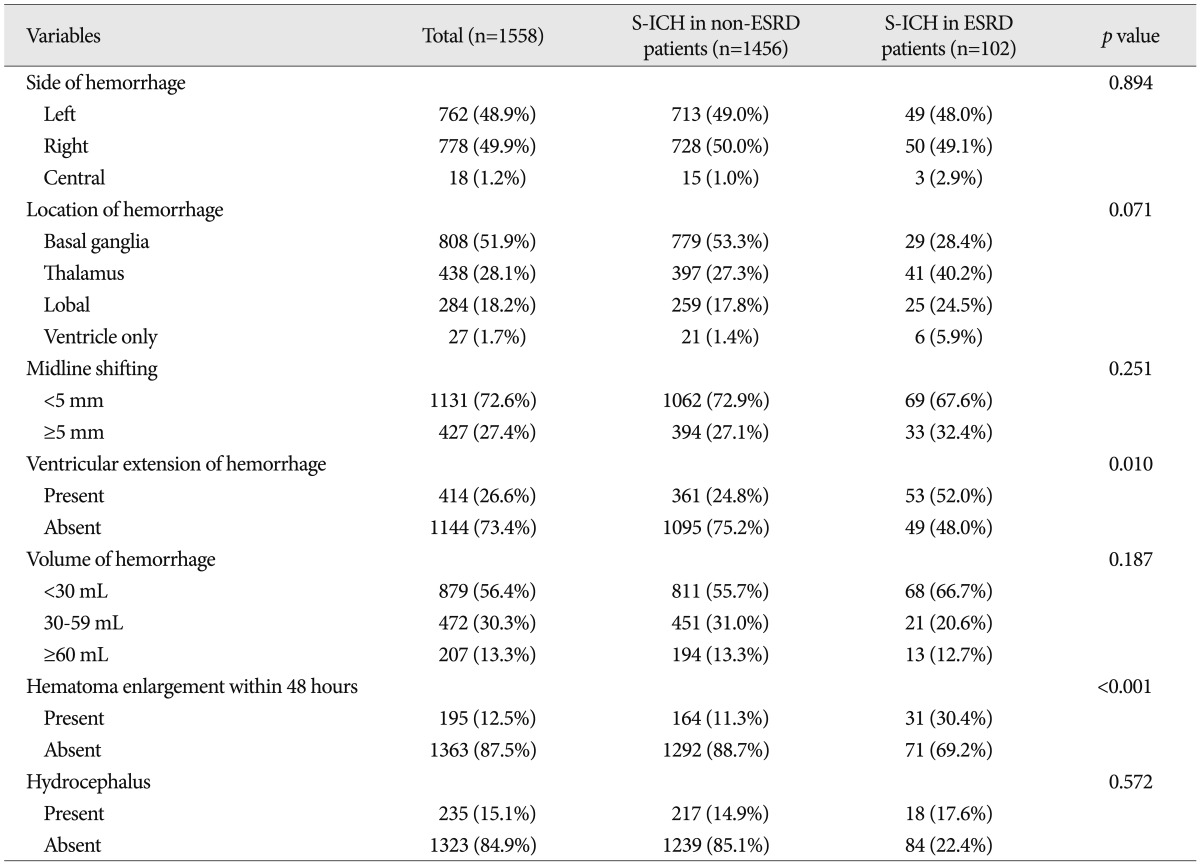
Table┬Ā3
Laboratory characteristics of patients with a spontaneous intracerebral hemorrhage 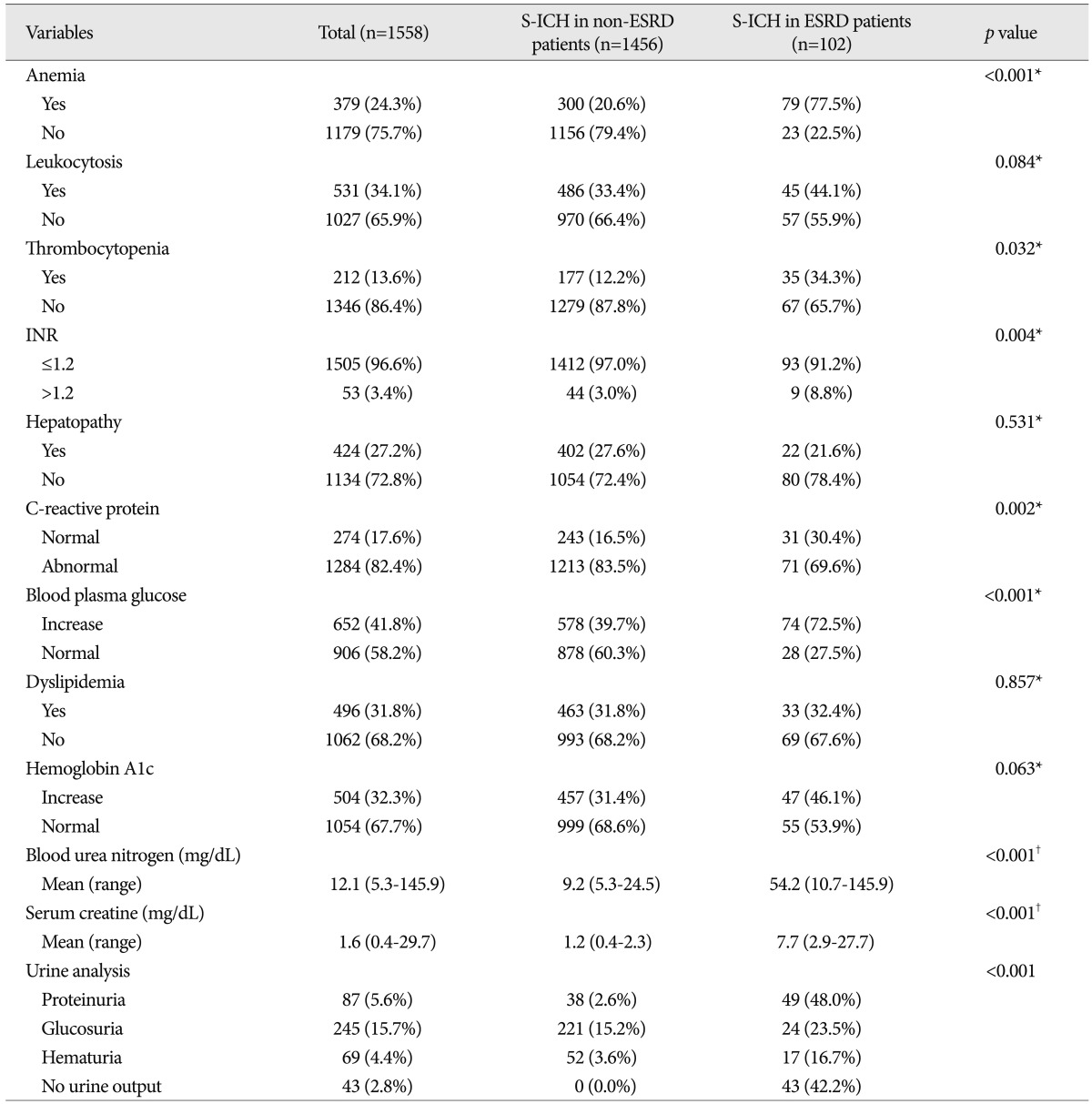
Table┬Ā4
Comparison of treatment modalities used for spontaneous intracerebral hemorrhage between patients with and without ESRD 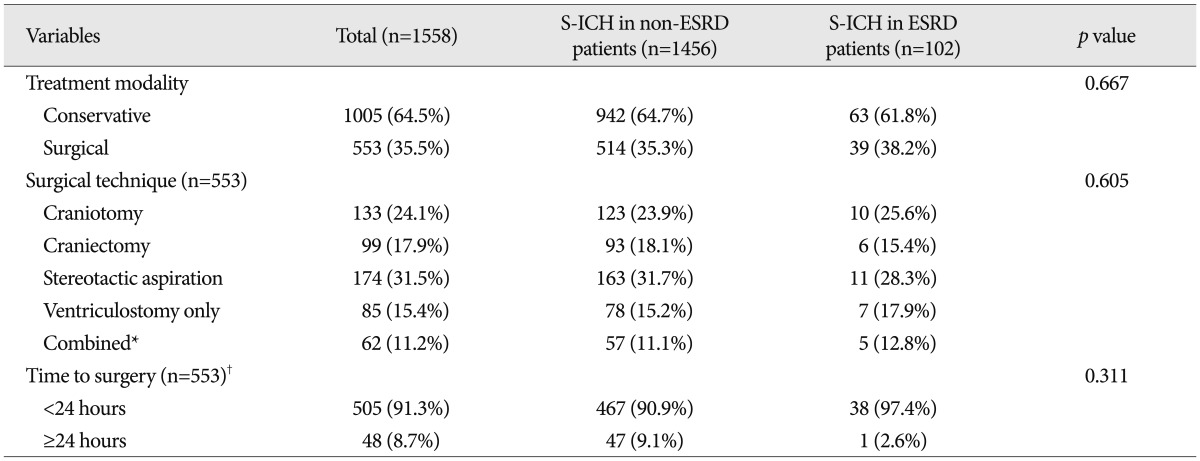
Table┬Ā5
Comparison of mortalities and functional outcome of for S-ICH between patients with and without ESRD 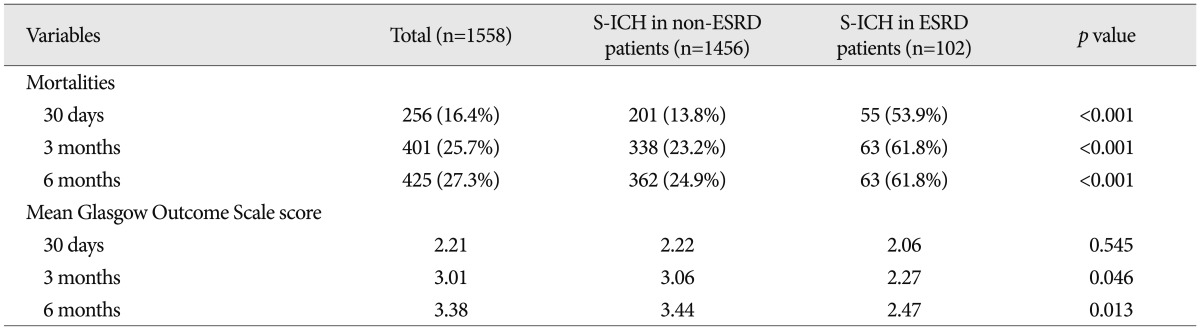
Table┬Ā6
Multivariate analysis of factors predictive of 30-day mortality after S-ICH using a logistic regression model 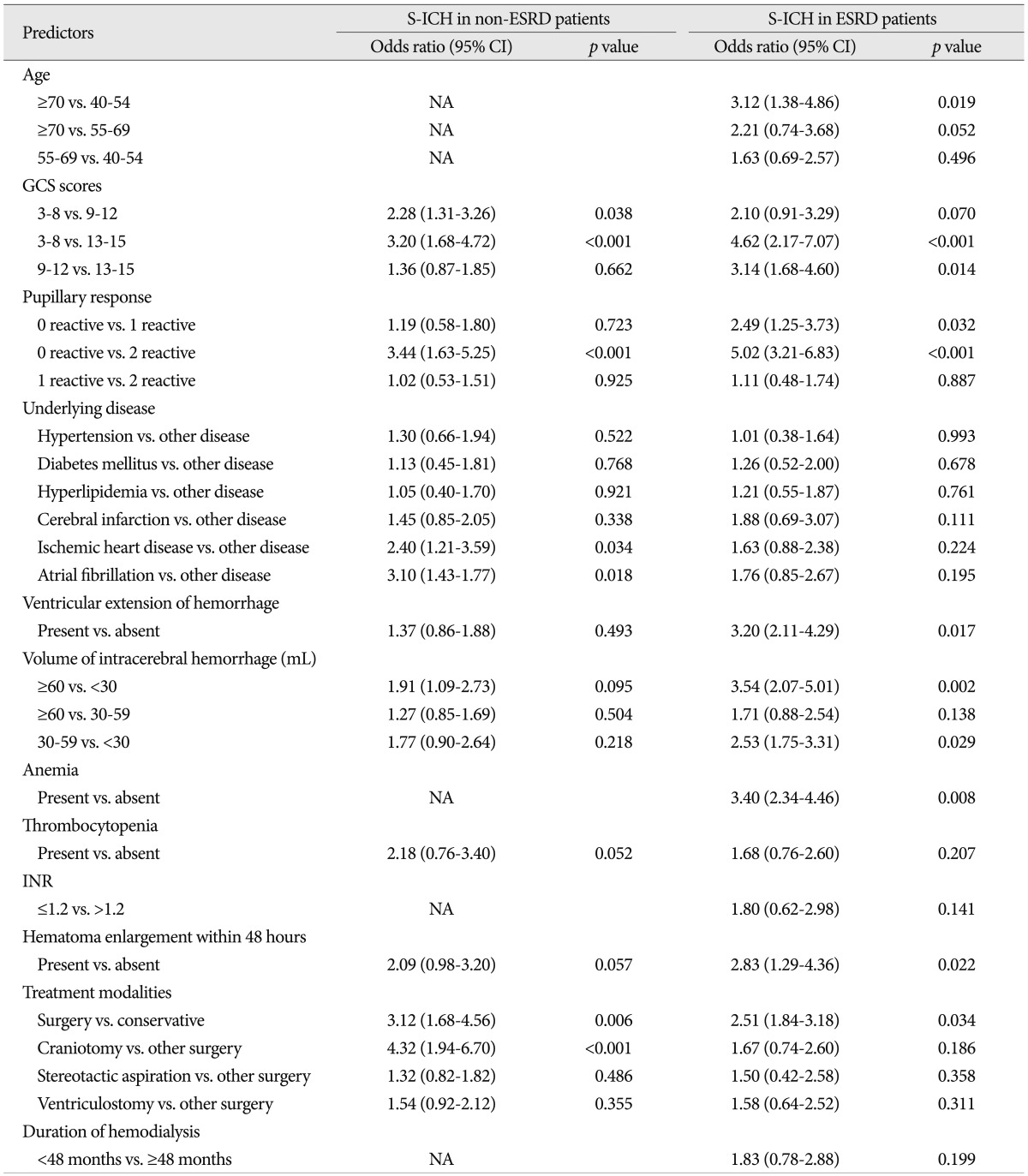
Table┬Ā7
Multivariate analysis of factors predictive of functional recovery at 6 months after S-ICH 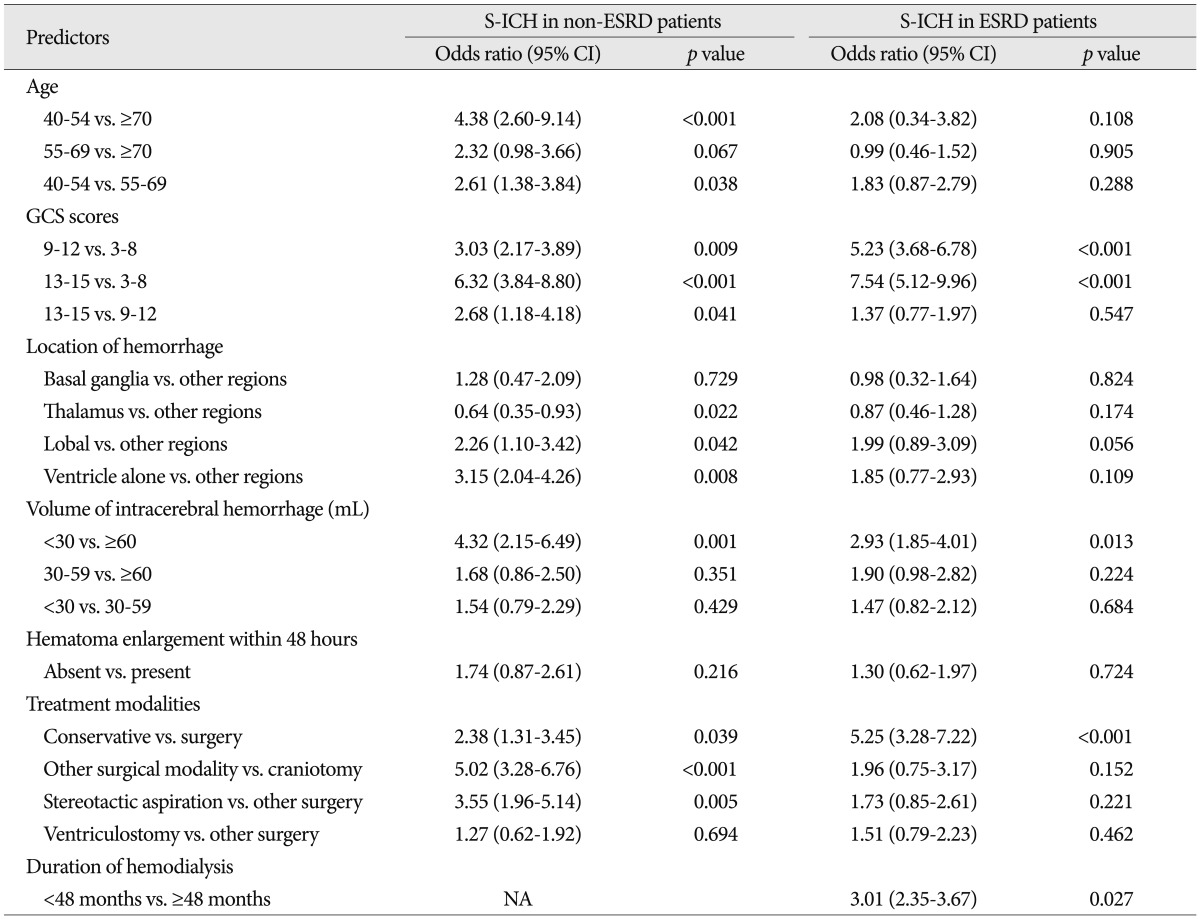
|
|






















