INTRODUCTION
The sinking skin flap syndrome is a rare complication after a large craniectomy. It consists of a sunken skin above the bone defect with neurological symptoms such as severe headache, mental changes, focal deficits, or seizures. The sinking skin flap syndrome may progress to "paradoxical herniation" as a consequence of the atmospheric pressure exceeding intracranial pressure and may eventually lead to coma and death6).
Cranioplasty is mostly required to treat the sinking skin flap syndrome to achieve further neurological improvement1). In this case report, we report the occurrence of large intracerebral hemorrhage (ICH) at contralateral side and intraventricular hemorrhage (IVH) following cranioplasty with sinking skin flap syndrome. We also discuss about the mechanism of occurance of contralateral ICH after cranioplasty in a patient with sinking skin flap syndrome.
CASE REPORT
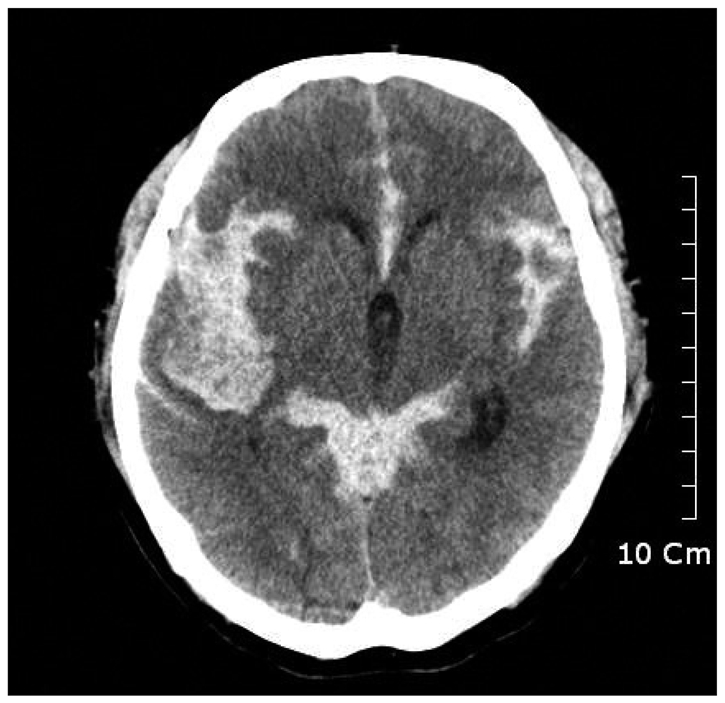
A 63-year-old male was admitted to our emergency care center with sudden onset of stuporous mental change and left side hemiparesis. A brain computed tomography (CT) revealed subarachnoid hemorrhage with IVH in whole ventricle, and right temporal lobe ICH. Following brain CT angiography displayed aneurysmal sac at right middle cerebral artery bifurcation (Fig. 1).
Emergent decompressive craniectomy and aneurysmal neck clipping have done. Left side motor function was improved immediately after surgery. After 3 weeks of surgery, cardiac arrest occurred. We could not reveal the cause of the arrest, and his motor function deteriorated to quadriparesis. Moreover, he had suffered from intractable pneumonia since the attack.
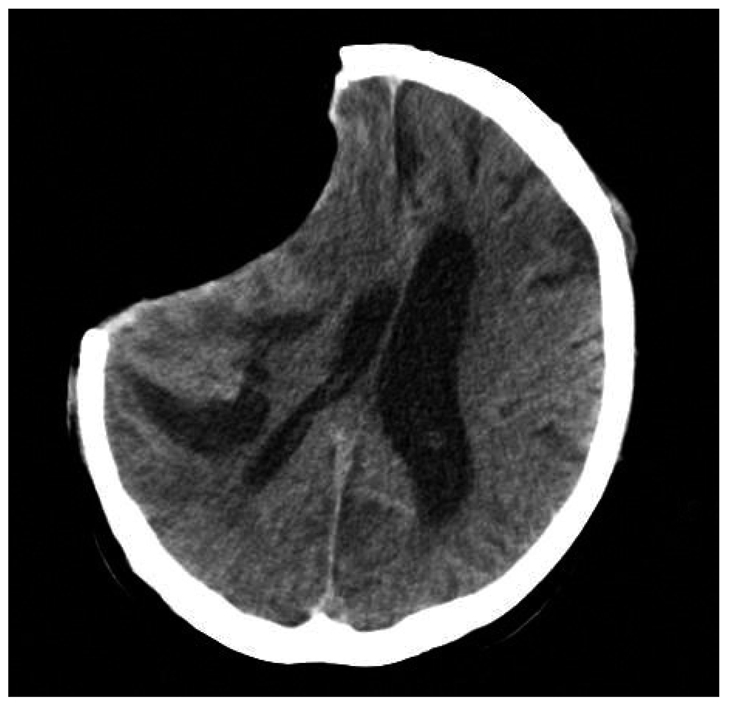
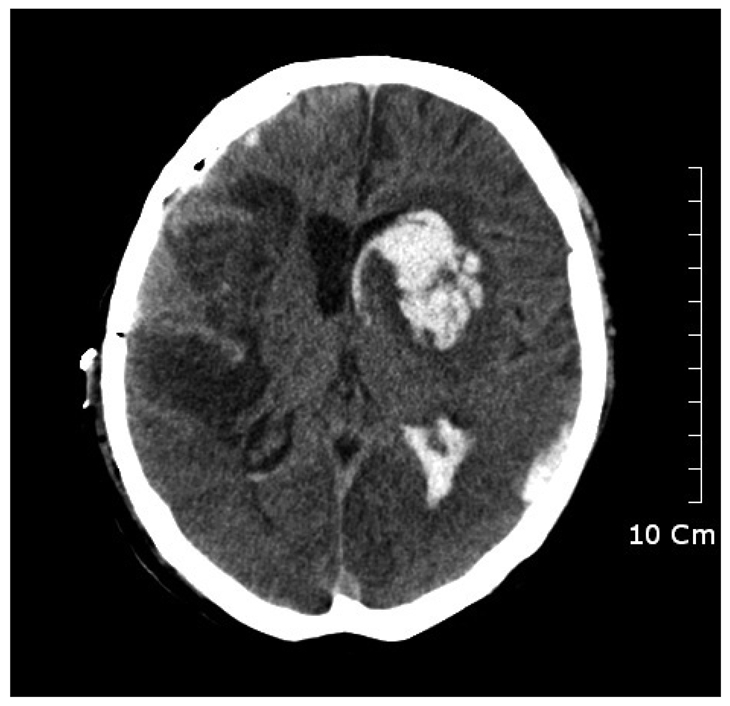
After 2 months of the craniotomy, follow up CT scan seen significant depression of the soft tissue flap with shifting of the midline (Fig. 2). The diagnosis of sinking flap syndrome was made and cranioplasty was done. At the day of surgery, he suddenly developed seizure of general tonic-clonic type about 4 hours after complete surgery. We checked his vital signs each 15 minutes for 1 hour, and then 30 minutes for 2 hours after surgery, there was no significant change in his blood pressure or heart rate. Emergency CT scan was performed and ICH at contralateral side of cranioplasty and IVH was detected (Fig. 3). We decided to avoid further aggressive therapy and controlled increased intracranial pressure (ICP) by using osmotic diuretics and antiepileptic drugs. And till now, he has no other complications of surgery, and was transferred to general ward.
DISCUSSION
Decompressive craniectomy is often performed to relieve ICP in patients with elevated ICP by traumatic head injury, stroke, and other causes that can increase ICP3).
Sinking skin flap syndrome is one of the complications of craniectomy, defined as serious disabling neurologic deficits and impairment of the general status with concave deformity and relaxation of the skin flap, which develops several weeks to months after large craniectomy.
Neurological symptoms such as headaches, mental changes, and language or motor deficits have been reported in sinking skin flap syndrome6). After craniectomy, the cranium does not maintain rigid structure. Increased transmission of atmospheric pressure will decrease volume of brain or CSF in patients with craniectomy2).
There have been a few studies about the neurological improvement after cranioplasty in patients with craniectomy. Isago et al.4) reported that in their case, the cerebral blood flow (CBF) was increased after cranioplasty measured by Xenon CT scan. Richaud et al.5) reported that in their all cases, there was a 15 to 30% increase in CBF in the area of cortex of cranioplasty. They proposed restoration of normal cerebral hemodynamics as a mechanism for neurological recovery after cranioplasty. These reports suggest that decreased CBF may be one of the underlying mechanisms by which sinking skin flap syndrome causes focal or diffuse neurological deficits. In our patient, seizure occurred about 4 hours after surgery. We did not perform immediate postoperative CT scan, but it can be inferred that the hemorrhage could be related to a sudden increase of CBF after cranioplasty.
Yoshida et al.7) reported significant increase of CBF and cerebral energy metabolism after cranioplasty by using 133Xenon CT and 31P Magnetic resonance spectroscopy. Other study results account for increase of CBF after cranioplasty, but none of them suggest the complications by increased flow, especially reperfusion injury.
In this case, CBF and cerebral metabolism decreased by sinking skin flap syndrome, and it may cause the deterioration of autoregulation of brain itself.
The authors suggest that a damaged hemisphere of brain slowly progressed to cerebromalacia. Its elastance might be lowered, so that pressure change might have followed by volume change would be fewer than contralateral side after cranioplasty. On the contrary, non-damaged side of brain relatively preserved its elastance, the pressure change of its parenchyme could be much more influenced than the damaged hemisphere.
Under this state, in our opinion, cerebral vascular resistance was decreased by expansion of brain after cranioplasty, cerebral perfusion pressure would be multiplied. And then CBF has restored suddenly and reperfusion injury from hyperperfusion, such as ICH, IVH have occurred.
We described sudden cerebral hemorrhage after cranioplasty in patient with sinking skin flap syndrome. It may arise from diminished autoregulation of brain due to decreased CBF and cerebral metabolism thus becoming unable to endure sudden hyperperfusion. Therefore, we should remember that reperfusion injury can be take place after cranioplasty in a patient with large skull defect and paradoxical herniation.
CONCLUSION
This is a case report of unusual complication of cranioplasty that occurred in a patient with sinking skin flap syndrome. Many reports suggest that decreased cerebral blood flow is an important mechanism of symptoms of sinking skin flap syndrome. ICH in this case might have caused by from reperfusion and recanalization of cerebral vessels with whose autoregulating system is unstable due to hypoxic damage of brain. We should consider the reperfusion injury of damaged brain after cranioplasty and determinate timely fashion of the surgery.