Choi, Park, Kim, Cha, Huh, and Kang: Direct Relationship between Angiographic Characteristics of Carotid Atherosclerotic Plaque and Filling Defect in the Cerebral Protection Filters : Based on the Conventional Angiography
Abstract
Objective
Neurologic complications during carotid artery stenting (CAS) are usually associated with distal embolic event. These embolic incident during CAS are highly associated with the carotid plaque instability. The current study was undertaken to identify the angiographic characteristics of carotid plaque vulnerability, which was represented as filling defect in the cerebral protection filters during CAS.
Methods
A total of 107 patients underwent CAS with use of a distal protection filter. Angiographic carotid plaque surface morphology was classified as smooth, irregular, and ulcerated. To determine predictable factors of filling defect in the protection filters, 11 variables were retrospectively analyzed which might influence filling defect in the protection filters during CAS.
Results
Filling defects during CAS were presented in the 33 cerebral protection filters. In multivariate analysis, angiographic ulceration [odds ratio (OR), 6.60; 95% confidence interval (CI) : 2.24, 19.4; p=0.001], higher stenosis degree (OR, 1.06; 95% CI : 1.00, 1.12; p=0.039), and coexistent thrombus (OR, 7.58; 95% CI : 1.69, 34.05; p=0.08) were highly associated with filling defect in the cerebral protection devices during CAS. Among several variables, angiographic surface ulceration was the only significant factor associated with flow stagnation during CAS (OR, 4.11; 95% CI : 1.33, 12.72; p=0.014).
Conclusion
Plaque surface morphology on carotid angiography can be a highly sensitive marker of plaque instability during CAS. The independent risk factors for filling defect in the filter devices during CAS were plaque ulceration, stenosis degree, and coexistent thrombus.
Key Words: Carotid artery В· Angiography В· Plaque.
INTRODUCTION
Carotid artery stenting (CAS) is recognized as a feasible alternative to carotid endarterectomy (CEA) for the management of carotid stenosis, because it is less invasive, less traumatic, and sometimes safer treatment modality in the patients with high risk of CEA 10,20). Although many advantages of CAS have been reported, one of its major concern is the occurrence of distal embolization during the procedure. Cerebral embolization during stenting procedure, which have potential risk of symptomatic stroke. Therefore, to minimize these problem, cerebral protection devices have been designed and applied to the prevention of distal embolization.
Periprocedural neurologic complication during CAS are associated with the size of embolus, and embolic incident during CAS are associated with the plaque vulnerability, such as lipid pool and intra-plaque hemorrhage 12-14,16). Up to recently, the vulnerability of carotid atherosclerotic plaque has been mostly focused about non-invasive imaging assessment, such as magnetic resonance image and ultrasound. Inversely, the conventional angiographic assessment of plaque vulnerability has been relatively neglected, because non-invasive imaging modalities are better examinations to evaluate the plaque composition that is associated with plaque vulnerability. However, conventional angiography is essential study prior to CAS, and it is gold standard modality to evaluate about carotid stenosis. The goal of this study is to determine if certain angiographic charaterisitics of carotid plaque were predictive of the vulnerable carotid atherosclerotic plaque, which was presented as filling defect in the cerebral protection filter during CAS.
MATERIALS AND METHODS
Patients
During 4 years (between January 2008 and December 2011), 107 patients (92 men and 11 women, 49 to 82 years old) underwent CAS in our institution. Indications for CAS included the patients with 1) symptomatic carotid stenosis вүҘ70%, or 2) asymptomatic carotid stenosis вүҘ80%, determined with duplex ultra-sound scan and confirmed with digital subtraction angiography. Measurement of angiographic carotid stenosis (percentage by diameter) were performed according to North American Symptomatic Carotid Endarterectomy methodology 2).
The evaluation of carotid atherosclerotic plaque
All patients underwent preoperative duplex ultrasound scanning and magnetic resonance angiography with complete neurologic examination by a neurologist. These tests were used to determine the degree of stenosis, rule out coexistent proximal or distal disease, and assess the lesion calcification, thrombus, and ulceration. All finding were subsequently confirmed by digital subtraction angiography. Baseline and preprocedural angiography was performed with the use of the automated analysis system coordinated with the angiographic equipment. Angiographic carotid atherosclerotic plaque surface was classified as smooth, irregular, ulcerated, and intraluminal free thrombus with underlying carotid stenosis ( Fig. 1). Plaques were classified as ulcerated if it fulfilled radiographic criteria of ulcer niche, seen in profile as a crater penetrating into a stenotic plaque and (when visible) double density on "en face" view. Plaques were classified as irregular if the plaque showed surface irregularity or multiple small possible craters or when there was difficulty distinguishing a real crater from normal wall between two plaque. Stenotic lesion length was classified as long lesion and short lesion, according to the basis of 1 cm. All stenotic lesions were limited to the cervical segment of internal carotid artery. Location of stenotic lesion was classified as carotid bulb involved lesion and restricted ascending cervical segment lesion without bulb involvement.
Carotid artery stenting procedure
Patients were either given aspirin 100 mg and clopidogrel 300 mg 12 hours prior to CAS or were treated with aspirin 100 mg and clopidogrel 75 mg daily before at least 3 days. All CAS procedure were performed from femoral approach using local anesthesia and intravenous sedation. A 6 Fr Shuttle sheath (Cook Inc., Bloomington, IN, USA) was placed into the descending thoracic aorta via the common femoral artery and then all patients were heparinized to maintain an activated clotting time with a target of at least 300 seconds. The target common carotid artery was cannulated using a hydrophilic guide wire over 6.5 Cook selective catheter (JBI, H1, Vitek) and the Shuttle sheath was advanced into the target common carotid artery. The side-arm of the guiding catheter was continuously flushed with pressurized heparinized normal saline. A filter-type protection device was carefully navigated across the stenotic lesion and deployed distally (at the entrance of carotid canal). Pre-stent deployment balloon angioplasty (predilatation) was performed and then a self-expanding stent was deployed in the stenotic artery. Postdilataion was selectively performed if residual stenosis after stent placement was over than 30%. Completion angiograms, including cervical and cerebral view, and 20-min post-stent angiogram for screening of acute in-stent thrombosis were routinely obtained.
We evaluated filling defect in the protection devices which suspected thromboembolic materials captured by the filter-type protection devices and flow stagnation at the filter level was seen immediately after balloon angioplasty or stent deployment ( Fig. 2).
Equipment
Only self-expanding stents were used. The types of stent deployed were open cell type in 96 cases (89.7%) and closed cell type in 11 cases (10.3%). Three different commercially available filter devices were used during CAS; Spider FX (ev3), 49 cases (45.8%); FilterWire EZ (Boston Scientific), 23 cases (21.5%); and Angioguard RX (Cordis), 35 cases (32.7%).
Statistical analysis
To determine the predictable factors of filling defect in the protection filters, 11 variables were retrospectively analyzed because it was believed that they might influence filling defect in the protection filters during CAS. The variables were classified as patient characteristics, angiographic characteristics, and equipment types. Each of three variable groups included sex, age, ulcer, irregularity, stenosis degree (categorical variable and continuous variable), stenosis length, stenosis location, coexistent thrombus, filter type, and stent type.
Patient characteristics, angiographic characteristics, and equipment types were summarized as a whole, as well as described specifically for subgroups by descriptive statistics. After descriptive analysis were performed, a Fisher's exact chi square test was used to compare categorical variables between groups, while a Student t-test was used to compare continuous variables (age, stenosis degree) between groups. Continuous variables appeared as mean values with SDs and categorical variables as frequencies with percentages.
Significant predictors for filling defect in the filter devices during CAS were identified by using univariate analysis. Significant predictors were included in a multivariate analysis to determine the most significant risk factors for filling defect in the filter devices during CAS.
Odds ratio (OR) for comparison of two groups was summarized with its 95% confidence interval (CI) and p value using logistic regression. The multivariate model was created using a backward elimination method, and the probability was set at 0.10 for removal. ORs were also adjusted for factors affecting the response variable. p values lower than 0.05 were considered statistically significant. All statistical analysis were carried out using SAS version 9.1 statistical software.
RESULTS
The patient characteristics, angiographic characteristics, and equipment types were shown in Table 1. In all 107 protected CAS procedures, the filter device could be placed and at the end of the procedure retrieved successfully, and there was no procedure-related neurologic complication. During CAS, 33 cases showed filling defect in the protection filters (30.8%). The frequency of filling defect in the protection filters for each variable was shown in Table 1. Among several variables, only the degrees of carotid artery stenosis were analyzed by categorical and continuous variables. It was to evaluate a correlation between filling defect in the protection filters and continuous increase of stenosis (per one percentage). Patient characteristics and angiographic characteristics were analyzed on the univariate analysis, except for stenosis degree by categorical variables and equipment types. In univariate analysis, ulcer, stenosis degree [continuous variable (%)], surface irregularity and coexistent thrombus were found to be significant factors affecting filling defect in the filter devices ( p<0.05) ( Table 2). Odds of causing filling defect was about 9 times higher among patients with thrombus than among patients without thrombus (OR=8.88, p=0.002). Age, stenosis length, and stenosis location were not significantly associated with the filling defects in the protection filters. In multivariate analysis, as shown in Table 3, ulcer, stenosis degree, and thrombus were selected as statistically significant factors associated with the filling defect in the filter devices. Patients with ulcerated carotid plaque (28 of 107 cases, 26.2%) had filling defect in the protection filters significantly more frequently than did patients without ulcerated plaque (OR, 6.60; 95% CI : 2.24, 19.4; p=0.001). Patients with higher stenosis degree had filling defect in the protection filters significantly more frequently than did patients with lower stenosis degree (OR, 1.06; 95% CI : 1.00, 1.12; p=0.039). Patients with coexistent thrombus (12 of 107 cases, 11.2%) had filling defect in the filter devices significantly more frequently than did patients without thrombus (OR, 7.58; 95% CI : 1.69, 34.05; p=0.08). The relationship between patients and angiographic characteristics and flow stagnation during CAS were also analyzed on the uni- and multivariate analysis ( Table 4). Among several variables, angiographic surface ulceration was the only significant factor associated with flow stagnation during CAS. In multivariate analysis, the patients with ulcerated plaque showed 4 times higher rate of flow stagnation during CAS ( Table 5, 6).
DISCUSSION
Although CEA has been the primary treatment option for carotid stenosis, CAS has now been highlighted as an alternative treatment because it is less invasive and it has larger capability for high risk patients than CEA. More recently, the Carotid Revascularization Endarterectomy versus Stenting trial, did not reveal any significant difference between CAS and CEA among standard-risk patients. However, cerebral embolization has remained major drawback in CAS, which potentially can result in symptom of stroke 8). The objective of this study was to identify angiographic characteristics of carotid plaque that may affect plaque vulnerability, which was represented as filling defect in the cerebral protection filter during CAS, and we have shown that plaque surface morphology on angiography is strongly associated with plaque vulnerability. On the basis of univariate analysis, ulcer, stenosis degree (continuous variable), surface irregularity and coexistent thrombus were found to be statistically significant risk factors for filling defect in the filter devices during CAS. Multivariate analysis demonstrated that all of these variables were independent risk factors except surface irregularity.
Stenosis degree
We observed that increasing stenosis degree was associated with increased chance of filling defect in the protection filter. Previous experimental study has demonstrated a direct relationship between the risk macroembolization and degree of stenosis in a carotid plaque model 12). It showed that the severity of stenosis correlated with the number of embolic particles recovered after balloon angioplasty and stenting. Balloon angioplasty and stenting involve radial compression and fracture of plaque to increase luminal diameter. Higher compression pressures are required to displace plaque within lesions with greater stenosis. It can be expected that a high compression pressure applied to severely stenosed plaque contributed to an increased incidence of embolic particles. In addition, high blood flow velocity on stenotic plaque that is associated with a high proportion of hemorrhagic plaques 1). High flow velocity induce shear stresses in the arterial wall as a result of the Bernoulli effect which lead to hemorrhage in the wall 1,4,11). Therefore, a stenotic plaque may be a vulnerable. In our data, uni- and multivariate analysis the degree of carotid stenosis (continuous variables) was significant factor of showing filling defect during CAS.
Thrombus
Coexistent thrombus of carotid plaque is associated with rupture or erosion of the lesion, and it is one feature of vulnerable plaque 7,11). Any form of endothelial denudation leads to activation of the coagulation system due to exposition of highly thrombogenic plaque constitutents (lipids, tissue factor, collagens) to the blood stream 7,11,18). This thrombus is prone to dislodge from plaque, and present as filling defect in the filter device because of guide wire passage, balloon and stent expansion. Clinically, carotid stenosis with intraluminal thrombus is also associated with a poor prognosis. In the North American Symptomatic Carotid Endarterectomy Trial, the 30-day risk of stroke or death for medically treated patients with clot was 10.7% versus 2.2% for patients without clot 19). Also, the risk of surgical or endovascular treatment has been considered higher than patients without intraluminal thrombus. In our study, the presence of intraluminal thrombus showed the highest OR for filling defect in the filter device during CAS. In the multivariate analysis the patients with intraluminal thrombus showed about 7.5 times higher rate of distal embolic events than the patients without intraluminal thrombus.
Plaque ulceration
Plaque ulceration on angiography was well-known powerful predictor of subsequent stroke 3,17). Lovett et al. 6) described ulcerated plaque had higher OR for definite plaque rupture, large lipid core, less fibrous tissue and intraplaque hemorrhage than smooth plaque in their histological evaluation of carotid plaque surface morphology on angiography. And plaque ulceration had strongest association with overall plaque instability. In our study, the presence of ulceration of stenotic lesion on angiography has shown to be a strong independent predictor of distal embolic event. Furthermore, in uni- and multivariate analysis, ulceration was unique independent factor which made flow stagnation during CAS. In the multivariate analysis, the patients with plaque ulceration showed 4 times higher rate of flow stagnation during CAS than the patients without plaque ulceration. It means plaque ulceration is not only simply associated with distal embolic event but also associated with large amount of emboli which can make flow stagnation during CAS. This result shows the patients with carotid plaque ulceration have higher risk of macroembolic complication and once more explain why the plaque ulceration is such a powerful predictor of ipsilateral stroke in the patients suffer from carotid stenosis.
In the previous studies, some authors reported longer lesions and the stenosis involved carotid bulb were risk factors for thromboembolic complications during CAS 5,9,15). Longer lesions have a larger atherosclerotic "plaque burden" and carry a risk of dislodging embolic particles during the procedure. The reason for the increased thromboembolic complications with carotid bulb lesions are possible that carotid bulb lesions simply more difficult to initially engage with a guide wire compared with more distal lesions, and as a result have an increased risk of triggering embolic event. In our study, stenosis length and location were not statistically significant factors of filling defect in the protection filter. This study had some limitation. First, the applicability of this result to standard-risk patients is not definitive because the event of distal embolization may vary not only according to carotid plaque characteristics but also patient's medical and anatomic risk. A second potential limitation of this study is that all the carotid plaques included in our study were from selected patients were only limited CAS cases. Therefore, although it seems likely that the associations between angiographic surface morphology and plaque instability exists, our result are directly applicable only to the patients of CAS.
CONCLUSION
In conclusion, with the use of univariate and multivariate analysis, we have analyzed 11 variables to evaluate predictable factors for the vulnerable carotid atherosclerotic plaque, which was filling defect in the cerebral protection filters during CAS. The independent risk factors for filling defect in the filter devices during CAS were plaque ulceration, stenosis degree, and coexistent thrombus.
Acknowledgements
This work was supported by the Dong-A University Research Fund.
References
1. Beach KW, Hatsukami T, Detmer PR, Primozich JF, Ferguson MS, Gordon D, et al : Carotid artery intraplaque hemorrhage and stenotic velocity. Stroke 1993, 24 : 314-319,   2. North American Symptomatic Carotid Endarterectomy Trial CollaboratorsBeneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991, 325 : 445-453,   3. Eliasziw M, Streifler JY, Fox AJ, Hachinski VC, Ferguson GG, Barnett HJ : Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994, 25 : 304-308,   4. Holdsworth RJ, McCollum PT, Bryce JS, Harrison DK : Symptoms, stenosis and carotid plaque morphology. Is plaque morphology relevant? Eur J Vasc Endovasc Surg 1995, 9 : 80-85,   5. Krapf H, NГӨgele T, Kastrup A, BГјhring U, GrГ¶newГӨller E, Skalej M, et al : Risk factors for periprocedural complications in carotid artery stenting without filter protection : a serial diffusion-weighted MRI study. J Neurol 2006, 253 : 364-371,   6. Lovett JK, Gallagher PJ, Hands LJ, Walton J, Rothwell PM : Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation 2004, 110 : 2190-2197,   7. Lusis AJ : Atherosclerosis. Nature 2000, 407 : 233-241,    8. Mas JL, Chatellier G, Beyssen B : EVA-3S InvestigatorsCarotid angioplasty and stenting with and without cerebral protection : clinical alert from the Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) trial. Stroke 2004, 35 : e18-e20,  9. Mathur A, Roubin GS, Iyer SS, Piamsonboon C, Liu MW, Gomez CR, et al : Predictors of stroke complicating carotid artery stenting. Circulation 1998, 97 : 1239-1245,   10. Mozes G, Sullivan TM, Torres-Russotto DR, Bower TC, Hoskin TL, Sampaio SM, et al : Carotid endarterectomy in SAPPHIRE-eligible high-risk patients : implications for selecting patients for carotid angioplasty and stenting. J Vasc Surg 2004, 39 : 958-965; discussion 965-966,   11. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al : From vulnerable plaque to vulnerable patient : a call for new definitions and risk assessment strategies : Part I. Circulation 2003, 108 : 1664-1672,   12. Ohki T, Marin ML, Lyon RT, Berdejo GL, Soundararajan K, Ohki M, et al : Ex vivo human carotid artery bifurcation stenting : correlation of lesion characteristics with embolic potential. J Vasc Surg 1998, 27 : 463-471,   13. Parodi JC, La Mura R, Ferreira LM, Mendez MV, CersГіsimo H, SchГ¶nholz C, et al : Initial evaluation of carotid angioplasty and stenting with three different cerebral protection devices. J Vasc Surg 2000, 32 : 1127-1136,   14. Rapp JH, Pan XM, Sharp FR, Shah DM, Wille GA, Velez PM, et al : Atheroemboli to the brain : size threshold for causing acute neuronal cell death. J Vasc Surg 2000, 32 : 68-76,   15. Sayeed S, Stanziale SF, Wholey MH, Makaroun MS : Angiographic lesion characteristics can predict adverse outcomes after carotid artery stenting. J Vasc Surg 2008, 47 : 81-87,   16. TГјbler T, SchlГјter M, Dirsch O, Sievert H, BГ¶senberg I, Grube E, et al : Balloon-protected carotid artery stenting : relationship of periprocedural neurological complications with the size of particulate debris. Circulation 2001, 104 : 2791-2796,   17. Valton L, Larrue V, ArruГ© P, GГ©raud G, BГЁs A : Asymptomatic cerebral embolic signals in patients with carotid stenosis. Correlation with appearance of plaque ulceration on angiography. Stroke 1995, 26 : 813-815,   18. van der Wal AC, Becker AE : Atherosclerotic plaque rupture--pathologic basis of plaque stability and instability. Cardiovasc Res 1999, 41 : 334-344,   19. Villarreal J, Silva J, Eliasziw M, Sharpe B, Fox A, Hachinski V, et al : Trial NASCET. Prognosis of patients with intraluminal thrombus in the internal carotid artery. Stroke 1998, 29 : 276,
20. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al : Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004, 351 : 1493-1501,  
Fig.В 1
Angiographic carotid atherosclerotic plaque surface classification. A : Plaque with a crater penetrating into a stenotic plaque was classified ulcerative plaque. B : Plaque with just surface irregularity or multiple small possible crater was classified irregular surface plaque. C : Plaque with intraluminal free thrombus. 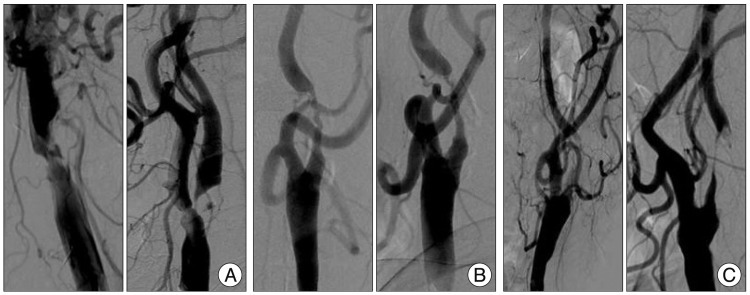
Fig.В 2
A : Digital subtraction angiography of post-stent assisted angioplasty shows filling defect in the filter device. B : Thromboembolic material captured by the filter device. 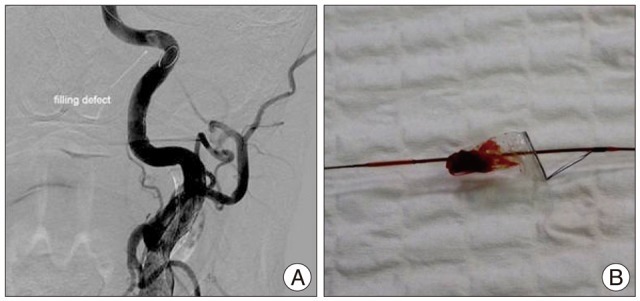
TableВ 1
Baseline characteristics of the study populations (filling defect in the cerebral protection devices) 
TableВ 2
Univariate analysis between variables and angiographic filling defect in the distal filter devices during carotid artery stenting 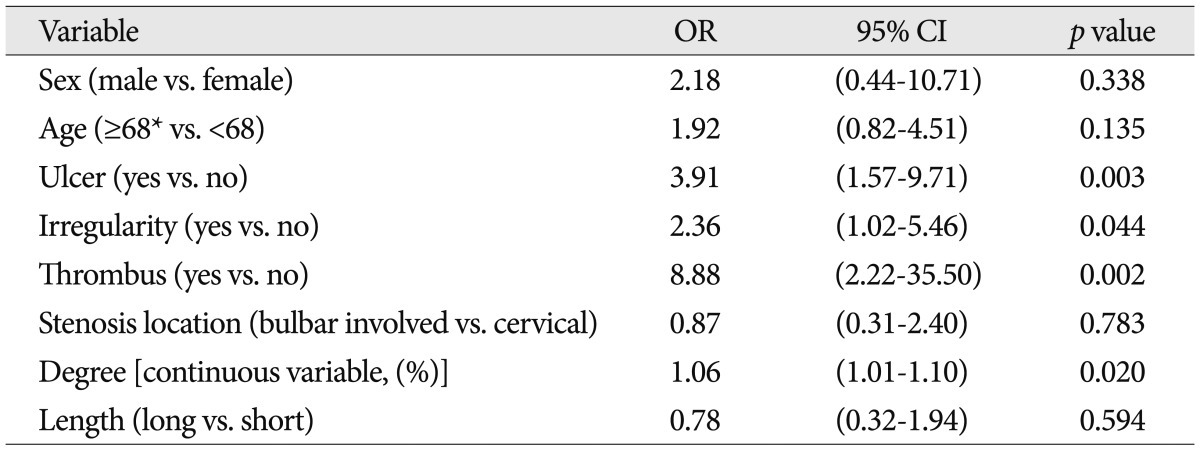
TableВ 3
Multivariate analysis between variables and angiographic filling defect in the distal filter devices during carotid artery stenting 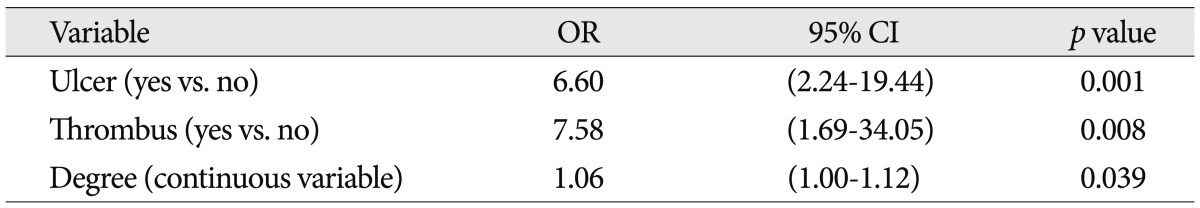
TableВ 4
Baseline characteristics of the study populations (flow stagnation) 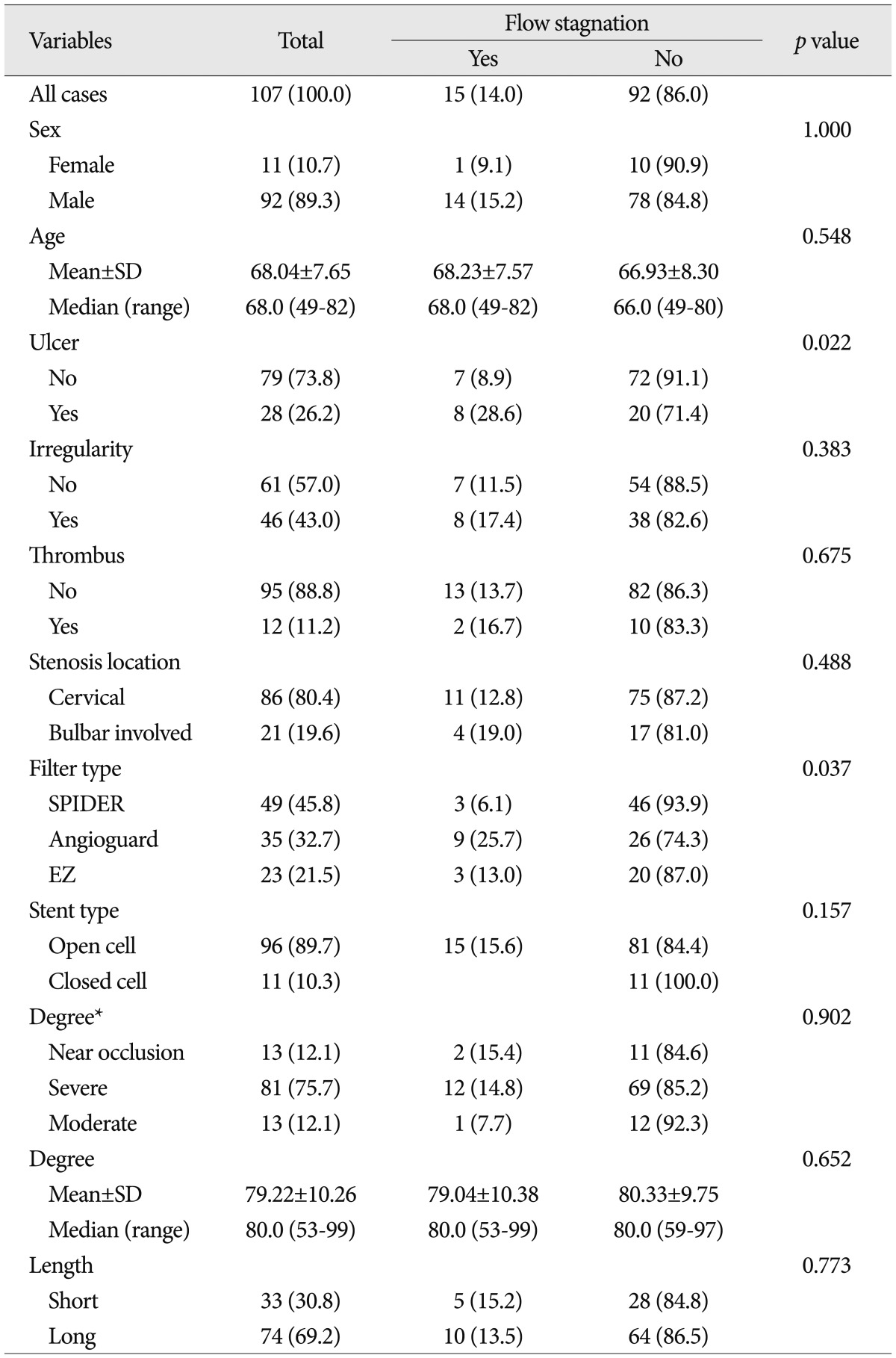
TableВ 5
Univariate analysis between variables and angiographic flow stagnation during carotid artery stenting 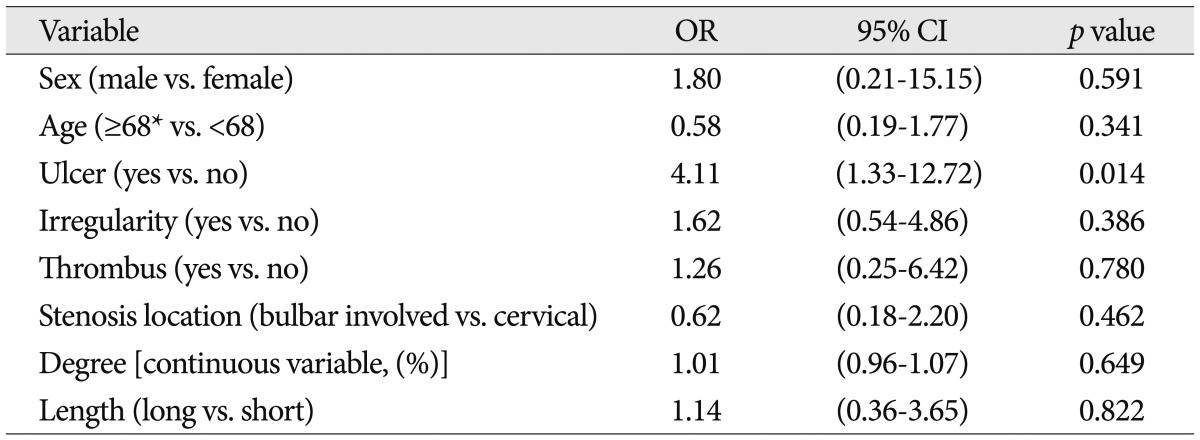
TableВ 6
Multivariate analysis between variables and angiographic flow stagnation during carotid artery stenting 
|
|























