INTRODUCTION
Brown tumors (BTs), also called as osteoclastomas, are rare nonneoplastic lesions that arise in the setting of hyperparathyroidism3,19). Hyperparathyroidism is overactivity of the parathyroid glands resulting in excess production of parathyroid hormone (PTH). PTH regulates calcium and phosphate metabolism of the body3). Excessive PTH secretion may be due to problems in the parathyroid glands such as adenomas, hyperplasia or carcinoma. This is known as primary hyperparathyroidism3,19). However, it may also occur in response to low calcium levels in such conditions as vitamin D deficiency or chronic renal disease that is known as secondary hyperparathyroidism3,19). BTs can arise as solitary or multiple lesions of any bone, more common in extremities, clavicle, ribs and pelvis. Spinal involvement is very rare1,23,45,67,89,1011,1213,1415,1617,1819,20).
In this study, we report a rare thoracic BT case that occured in the setting of primary hyperthyroidism. Tumor was treated succesfully by cooperation of endocrinology, general surgery and neurosurgery departments.
CASE REPORT
50-year-old man was admitted to our neurosurgery department with the chief complaint of difficulty in standing and walking due to leg weakness for nearly 2 days. He also described back pain while sitting and standing. Neurological examination demonstrated paraparesis with impaired anal sphincter tonus. He had no history of trauma or any systemic illness.
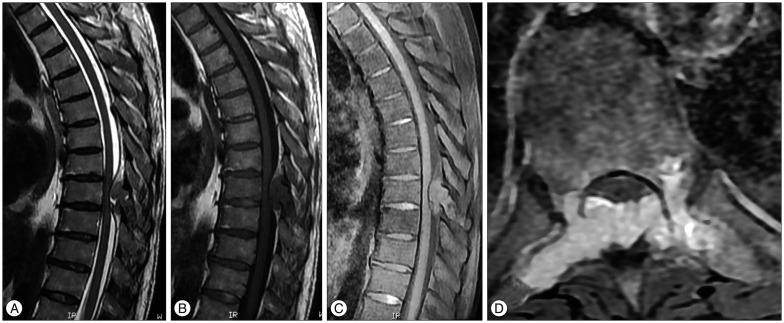
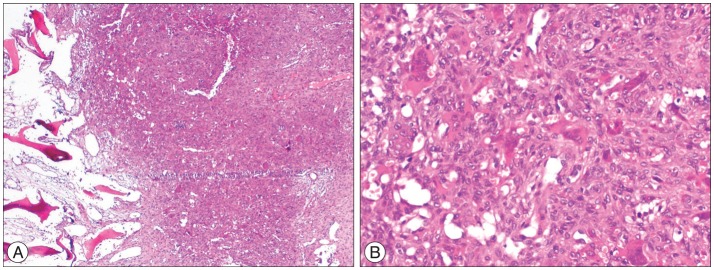
Thoracic magnetic resonance imaging (MRI) and computerized tomography (CT) revealed expansile mass lesion that was compressing the spinal cord at T9 level. Homogenously enhancing mass lesion was found to originate from the anterior portion of the spinous process and both laminas of T9 vertebra (Fig. 1). Routine blood tests were uneventful except a calcium value of 14.3 mg/dL (8.4-10.2). As blood parathormone (PTH) test also revealed a very high value of 547.45 pg/mL (15-68.3), endocrinology consultation was ordered to rule out primary hyperparathyroidism. Neck sonography demonstrated hyperplasia and multiple nodules on the thyroid gland. However, any cystic or solid pathologic lesion compatible with adenoma were not seen on the parathyroid regions. Parathyroid scintigraphy with Tc-99m MIBI revealed focal activity retention on the inferior portion of the right thyroid lobe. Abdomen and thorax CT were uneventful. Endocrinology department insisted for the urgent parathyroidectomy in order to minimize hypercalcemia-related complications. So, patient with the diagnosis of primary hyperparathyroidism underwent surgery for parathyroidectomy first. Pathological parathyroid tissue was found and excised. Total thyroidectomy was also performed since frozen examination of a suspected thyroid nodule was reported as micropapillary carcinoma. The day after parathyroid and thyroid surgery blood calcium and PTH levels decreased to 10.2 mg/dL and 10.42 pg/mL, respectively. Patient underwent spine surgery. Tumor was excised in piecemeal fashion. Wide posterior decompression (T8 partial/T9 total laminectomy+bilateral T9 transvers process and pedicle excision) was followed by T8-10 posterior instrumentation and fusion (Fig. 2). Paraparesis resolved postoperatively. Patient was mobilised with a brace on postoperative day 1. Absolute pathology reports were consistent with the parathyroid adenoma and spinal Brown tumor (Fig. 3).
DISCUSSION
Brown tumors occur due to increased osteoclastic activity in the setting of either primary or secondary hyperparathyroidism3,819). Parathyroid adenomas or hyperplasia constitute the major BT source in primary hyperparathyroidism while chronic renal failure is the leading cause in secondary hyperparathyroidism3). BTs are localized form of osteitis fibrosa cystica (OFC) that is commonly associated with hyperparathyroidism. OFC is a process that is characterized by hyperstimulation of osteoclastic proliferation via longstanding excessive PTH production causing bone resorption and bone marrow fibrosis3,710,14).
Histopathologically, BTs are composed of osteoclast-like giant cells and hemosiderin with a fibrovascular stroma. The name Brown tumor comes from the accumulation of hemosiderin that gives the surrounding stroma a brown color3,8). BTs look like similar histologically to other giant cell lesions such as giant cell tumor, aneursymal bone cyst and reparative giant cell granuloma8). Only the clinical manifestation and endocrinologic status help to differentiate BTs from other giant cell lesions.
BTs usually develop in the third or fourth decades of life8). In the beginning, BTs that develop in primary hyperparathyroidism were seen much more common. However, they are seen more often recently as a result of secondary hyperparathyroidism that is closely related with increased life expectancy of chronic renal disease patients who require hemodialysis3,8). Most of the patients with the diagnosis of primary hyperparathyroidism present with kidney stones or isolated hypercalcemia3). However, nearly one third of patients are asymptomatic and hypercalcemia is found incidentally. Skeletal involvement such as bone resorption, bone cysts, BTs and osteopenia is seen on the late phase of hyperparathyroidism3,11).
The symptoms of spinal BTs include axial pain, radiculopathy, myelopathy and myeloradiculopathy according to their locations1,312,1314,1516,1718,1920). Radiographically, BTs do not have a pathognomonic appearance. It can mimic multiple myeloma, metastases, sarcomas and other giant cell lesions6,710,19). X-Rays demonstrate solitary or multiple, lytic, expansile lesions. Bone cortex might be thinned and expanded but it is not penetrated by the tumor frequently. On CT scan, relatively well demarcated soft tissue mass with local bone erosion and expansion is observed. MRI is the diagnosis modality of choice in evaluating the location, extent of spinal tumor and neural compression. On MRI, BTs are commonly seen hypointense on T1-weighted images, hypointense or hyperintense on T2-weighted images and homogenous enhancement is noted after contrast injection. Intratumoral hemorrhages lead to fluid-fluid level appearance on MRI3,819).
Plasmocytoma, lymphoma, giant cell tumors and metastates should be ruled out in the differential diagnosis of BTs3,68,19). When a solitary lesion that correspond to BT is found on the spine, differential diagnosis might include true giant cell tumor, aneurysmal bone cyst and giant cell reparative granuloma. Also, hyperparathyroidism associated bone changes such as loss of lamina dura of the teeth roots and generalized demineralization of the medullary bone of the jaw could help the surgeon to differentiate BT from other giant cell lesions8).
Treatment of BTs involve both the management of hyperparathyroidism and neural decompression. Total or subtotal parathyroidectomy is the gold standard for the treatment of primary hyperparathyroidism3,711,19). Parathyroid surgery rapidly decreases the excessive amount of PTH thus achieving complete regression of the lesions with remineralization3,46). Parathyroidectomy and medical treatment may also be used together. Neurological status is the main determinant in case of neural compression in the treatment of BTs3,7). Immediate surgical decompression could be necessary for achieving good outcome. Unless the decompression procedure does lead to instability, patients can be mobilized in braces without spinal fixation. However, spinal instrumentation and fusion is needed if the tumor is too large, affects multiple levels or pathological spinal fractures exist7,1012,19).
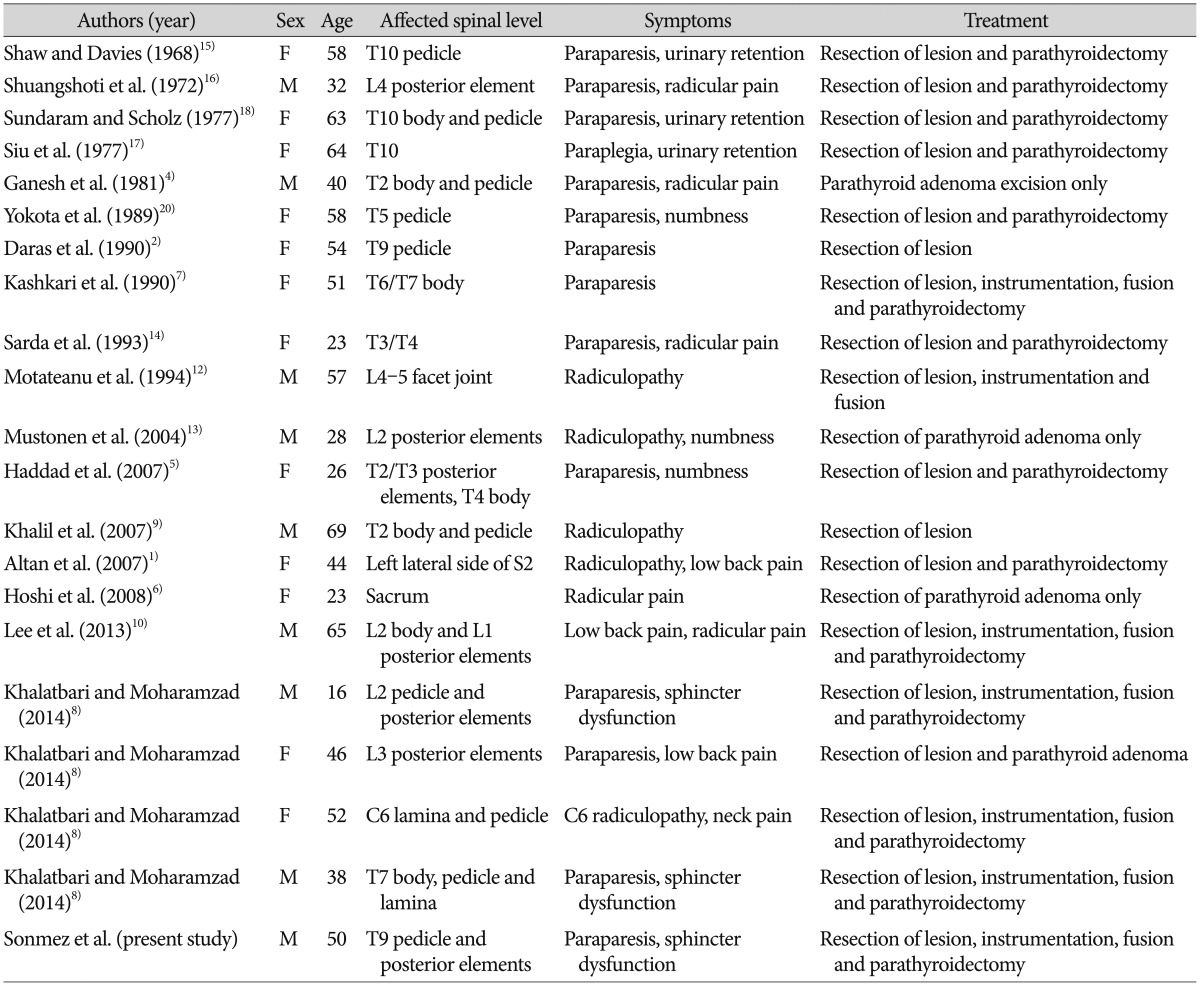
Our search in English literature demonstrated 20 spinal BT patients with primary hyperparathyroidism (Table 1). 12 of 20 patients (60%) were women. The patients' age ranged from 16 to 69 with a mean of 45.3 years. Thoracic spine was the most affected part of the spine (55%) followed by lumbar, sacral and cervical regions. Almost all the patients were presented with major neurologic deficit. 15 patients (75%) underwent double surgeries for both removing the mass and to treat the primary hyperparathyroidism. 6 patients (35%) underwent instrumentation and fusion surgery whilst 11 patients (65%) underwent only decompression surgery. After surgery, improvement of symptoms were observed in all patients.
CONCLUSION
BTs should be kept in mind in the differential diagnosis of lytic, expansile spinal tumors, especially with the histopathological diagnosis of giant cell tumors. Treatment consists of both treating the underlying primary pathology and decompression of the neural structures, if necessary. Urgent surgery can be needed to preserve neural function and stabilize the spine.