Yoon, Lho, and Chung: Evaluation of 3-Dimensional Exoscopes in Brain Tumor Surgery
Abstract
Objective
Though the operating microscope (OM) has been the standard optical system in neurosurgery, a new technology called three-dimensional (3D) exoscope has emerged as an alternative. Herein, two types of 3D exoscopes for brain tumor surgery are presented. In addition, the advantages and limitations compared with the OM are discussed.
Methods
In the present study, 3D exoscope VOMS-100 or VITOM 3D was used in 11 patients with brain tumor who underwent surgical resection; the Kinevo 900 OM was used only in emergency. After completion of all surgeries, the participants were surveyed with a questionnaire regarding video image quality on the display monitor, handling of equipment, ergonomics, educational usefulness, 3D glasses, and expectation as a substitute for the OM.
Results
Among 11 patients, nine patients underwent neurosurgical resection with only 3D exoscope; however, two patients required additional aid with the OM due to difficulty in hemostasis. Regarding video image quality, VITOM 3D was mostly equivalent to the OM, but VOMS-100 was not. However, both 3D exoscopes showed advantages in accessibility of instruments in the surgical field and occupied less space in the operating theater. Differences in ergonomics and educational usefulness between the exoscopes were not reported. Respondents did not experience discomfort in wearing 3D glasses and thought the exoscopes could be currently, and in the future, used as a substitute for the OM.
Conclusion
Although many neurosurgeons are not familiar with 3D exoscopes, they have advantages compared with the OM and similar image quality. Exoscopes could be a substitute for OM in the future if some limitations are overcome.
Key Words: 3D exoscope · Microscopy · Brain neoplasms · Surveys and questionnaires.
INTRODUCTION
Since the first introduction of the operating microscope (OM) for neurilemoma surgery in 1957, binocular microscopes have been considered essential equipment in the neurosurgical field [ 5]. The microscopes have also contributed to great advances in neurosurgical technique. Recently, OMs have been integrated with neuronavigation systems and offer intraoperative fluorescence imaging of vessels and tumors as well as good magnification, sufficient illumination, and highdefinition (HD) videos [ 15]. However, OMs are inherently limited by the ocular lens system and size. Surgeons often have to assume an uncomfortable posture for a long period of time while looking at the surgical field through a small lens eyepiece, causing eye fatigue and excessive tension in the body. In addition, the weight and size of OMs have gradually increased, limiting its mobility and increasing the space occupied in the operating theater. The surgical assistant’s view is not the same as that of the primary operating surgeon, and the observation tube or eyepiece lens for the assistant requires readjustment when the microscope is moved [ 7]. In addition, the current cost of an OM is more than 500 million KRW depending on the options, calling into questions the cost effectiveness. Exoscope is a piece of equipment that has a camera mounted outside the surgical field. In the early model, the image was acquired using a low-quality endoscopic system [ 2, 4]. However, with improvements in imaging and optical technology, a highresolution, three-dimensional (3D) video can be acquired and displayed. Therefore, the exoscope has emerged in the neurosurgical field as a new hybrid optical system between the OM and the endoscope [ 1, 10, 12]. However, many neurosurgeons are not yet familiar with the 3D exoscope and question whether it can be used as a substitute for OM. We used two types of 3D exoscopes, VOMS-100 (SOMETECH, Seoul, Korea) and VITOM 3D (Karl Storz GmbH, Tuttlingen, Germany) in brain tumor surgeries. Herein, we present the advantages and limitations of 3D exoscopes compared with the OM.
MATERAIALS AND METHODS
This study was approved by the Ethical Institutional Review Board (IRB) of the Catholic Medical Center in the Republic of Korea (IRB number, OC20RAS1010) and the informed consent was waived by the regulation of our IRB.
Patients and surgery
Exoscopes were used in brain tumor surgeries except for skull base tumors. The present study included 11 patients : three patients with metastatic brain tumor, three with meningioma, two with glioblastoma, one with anaplastic astrocytoma, one with anaplastic xanthoastrocytoma, and one with hemangioblastoma. Tumors were located at the supratentorial area in nine patients and in the cerebellum in the other two patients. Two tumors, one metastatic brain tumor and one glioblastoma, were located at the deep structure of the brain involving the corpus callosum, while the other nine tumors were located superficially. The median volume of tumors was 27.9 mL (range, 11.8-131). After the craniotomies were performed, exoscopes were used to resect tumors in all patients. VOMS-100 was used in five patients and VITOM 3D in the other six patients. Tumor resection was started under an exoscope; however, the OM, Kinevo 900 (Carl Zeiss, Oberkochen, Germany), was prepared for emergency if the surgery could not be further performed only with the exoscope ( Table 1 and Fig. 1).
Exoscopes
The VOMS-100 exoscope consists of three parts : a main single unit composed of the camera and the image console connected by an arm, the display monitor, and the foot panel switch. The arm has three joints that allow the exoscope to be manually moved freely horizontally and vertically. The head, including the camera and lens, can be moved like a pendulum within a limited range by the motorized system operated by the foot panel switch. The optical zoom can be doubled by turning the turret on the lens manually, and the digital zoom can be increased 3-fold by control of the foot panel switch. Five types of lenses can be interchanged with different working distances (210, 220, 230, 310, or 350 mm) and diagonal lengths (10-20, 19-38, 30-60, 30-55, or 50-90 mm). The camera captures the image in 3D with full HD (full-HD, 1920 ×1080 pixels). The full-HD monitor is displayed in front of the surgeon, and the operation is performed while watching the monitor and wearing 3D glasses. The VOMS-100 cannot be integrated with the neuronavigation system and does not have fluorescence detection. The weight of the equipment, excluding the monitor, is 95 kg, which is light and can be moved easily [ 14]. The VITOM 3D exoscope was described in many previous reports [ 1, 11, 13]. The exoscope is composed of five units : camera, control unit, holding arm, image console, and monitor. The camera requires connection with a light power console using a fiberoptic light cable similar to the endoscopic system. The optical zoom can be increased 4-fold by the motorized lens, and digital zoom can be increased up to 30 times without exchanging the lens. The exoscope has a wide working distance ranging from 200-500 mm and 4K image sensor chips, which can display a 3D video with high resolution (3840×2160 pixels) on the monitor. The control unit, IMAGE 1 PILOT, has four programmable function buttons and one jog-type wheel, which controls zoom, focusing, and view of field in 3D. However, the camera only can be moved manually. The control unit is mounted next to the operation table with the supporting arm. The holding arm, VERSACRANE TM (Karl Storz GmbH), has five joints for movement and the clamping jaw for holding the exoscope. The cables connecting the camera are also clamped to this holding arm. A 4K monitor is used to display the video of the operation performed while wearing 3D glasses, similar to the procedure with the VOMS-100. The VITOM 3D exoscope also cannot be integrated with a neuronavigation system and cannot detect fluorescence [ 6]. The technical specifications of exoscopes and OM were summarized on Table 2.
Questionnaire
After the exoscopic surgeries, a survey was administered to the participating two neurosurgeons, three residents and eight nurses. The questionnaire included 17 questions regarding the image quality on the display monitor, handling of equipment, ergonomics, educational usefulness, 3D glasses, and expectations. Thirteen questions were scored on a 5-point scale to compare the exoscopes with the OM, and the last four questions comprised selection of the one correct answer. The questions regarding image quality shown on the display monitor included the overall image quality, the image when bleeding appeared, and the image when the surgical field was magnified. Illumination of the overall surgical field including the deep area was also evaluated. Equipment handling was evaluated based on accessibility of instruments to the surgical field, focusing the image at the surgical field, convenience of preparation and installation, degree of space occupation in the operating theater, and overall convenience during surgery. The ergonomics were evaluated based on eye fatigue including body discomfort during the surgery. Whether the exoscope would be helpful for educational purposes was investigated. Because all surgical participants must wear 3D glasses, the preference between eyepiece lens and 3D glasses and the experience of dizziness while wearing 3D glasses were also evaluated. In addition, expectations regarding the possibility of replacing the OM with the exoscope currently and in the future were surveyed ( Table 3).
Statistical analysis
The results of the questions were compared using statistical analysis. Regarding the first 13 questions, the mean values of scored points for VOMS-100, VITOM 3D, and Kinevo 900 were calculated and independently compared using the Mann-Whitney U test. The last three questions were analyzed using the chi-square test to determine whether differences existed between yes and no answers. Statistical analyses were performed using the SPSS software package (version 18.0; IBM SPSS Statistics, Chicago, IL, USA), and p-values <0.05 were considered statistically significant.
RESULTS
Surgical results
Among total 11 patients, nine patients were completed the surgical resection of tumors with the exoscope only. However, two patients, one with metastatic brain tumor (case No. 6) and 1 with hemangioblastoma (case No. 12), required use of the OM because the bleeding source from small vessels was not clearly identified under the exoscope. Gross total resection was performed in nine patients and subtotal resection in two patients. Immediate postoperative complication of a supratentorial epidural hemorrhage occurred in the patient with cerebellar hemangioblastoma; however, the complication was not associated with the exoscopic system.
Response to questionnaire
Respondents scored the exoscopes relatively low regarding image quality on the monitor. In questions regarding overall image quality, VOMS-100, VITOM 3D, and Kinevo 900 had a mean score of 3.2, 3.9, and 4 points, respectively. A statistically significant difference was observed between VOMS-100 and Kinevo 900 ( p=0.01) but not between VITOM 3D and Kinevo 900 ( p=0.27). When the bleeding site was determined, VOMS-100, VITOM 3D, and Kinevo 900 scored a mean of 3.1, 3.5, and 4 points, respectively. Statistically, VOMS-100 was significantly inferior to Kinevo 900 ( p=0.04), however, the VITOM 3D was not different from Kinevo 900 ( p=0.21). When the image was magnified, VOMS-100, VITOM 3D, and Kinevo 900 scored a mean of 2.9, 3.4, and 4.2 points, respectively. Both exoscopes showed a statistical difference from the Kinevo 900 ( p=0.00 in VOMS-100; p=0.03 in VITOM 3D). In the question regarding overall brightness, VOMS-100, VITOM 3D, and Kinevo 900 scored a mean of 3.2, 3.8, and 4.3 points, respectively. The brightness of VOMS-100 was significantly lower than that of Kinevo 900 ( p=0.01), but that of the VITOM 3D was not ( p=0.21). In the question of brightness of deep surgical field, VOMS-100, VITOM 3D, and Kinevo 900 scored a mean of 3.1, 3.6, and 4 points, respectively. In addition, statistically significant difference was observed between VOMS100 and Kinevo 900 ( p=0.02) but not between VITOM 3D and Kinevo 900 ( p=0.22) ( Fig. 2A). In the question regarding accessibility of instruments in the surgical field, VOMS-100, VITOM 3D, and Kinevo 900 scored a mean of 3.9, 4.2, and 3.6 points, respectively. VITOM 3D showed a statistically significant difference compared with Kinevo 900 ( p=0.04) but not VOMS-100 ( p=0.46). Regarding the ease of focusing on the surgical field, VOMS-100, VITOM 3D, and Kinevo 900 scored a mean of 3.4, 3.6, and 4.4 points, respectively. A statistical difference was observed between the exoscopes and the OM ( p=0.00 in VOMS-100; p=0.02 in VITOM 3D). In the question regarding convenience of preparation and installation, VOMS-100, VITOM 3D, and Kinevo 900 scored a mean of 3.3, 3.5, and 3.2 points, respectively, and statistical difference was not observed between the exoscopes and the OM ( p=0.84 in VOMS-100; p=0.49 in VITOM 3D). Regarding the amount of space occupied in the operating theater, VOMS-100, VITOM 3D, and Kinevo 900 scored 3.1, 3.1, and 4.3 points, respectively. Both exoscopes received significantly lower scores compared with the OM ( p=0.00 in VOMS-100; p=0.00 in VITOM 3D). However, statistical difference was not observed between exoscopes and the OM in overall convenience during surgery; mean 3.8, 3.8, and 3.7 points for VOMS-100, VITOM 3D, and Kinevo 900, respectively ( p=0.89 in VOMS-100 vs. Kinevo 900, p=0.83 in VITOM 3D vs. Kinevo 900) ( Fig. 2B). In the question regarding eye fatigue during surgery, the VOMS-100, VITOM 3D, and Kinevo 900 scored a mean of 3.6, 3.6, and 3.8 points, respectively ( p=0.69 in VOMS-100 vs. Kinevo 900, p=0.78 in VITOM 3D vs. Kinevo 900). In the question regarding body discomfort during surgery, VOMS-100, VITOM 3D, and Kinevo 900 scored a mean of 2.8, 3.1, and 3.4 points, respectively, with no statistical difference ( p=0.22 in VOMS-100 vs. Kinevo 900, p=0.53 in VITOM 3D vs. Kinevo 900). In the question regarding use for educational purpose, VOMS-100, VITOM 3D, and Kinevo 900 received a mean of 3.8, 4, and 3.8 points, respectively, with no statistical difference between exoscopes and the OM ( p=0.81 in VOMS-100 vs. Kinevo 900, p=0.43 in VITOM 3D vs. Kinevo 900) ( Fig. 2C). Regarding the preference between eyepiece lens and 3D glasses, eight respondents chose 3D glasses, five selected eyepiece lenses, and five did not have a preference. Statistical difference was not observed among respondents regarding experience of dizziness when wearing 3D glasses ( p=0.84). In the question whether exoscopes can be a substitute for OM during brain tumor surgery, nine respondents answered it was currently possible, and 10 respondents answered it will be possible in the future ( Fig. 2D).
DISCUSSION
Exoscopic surgery evolved from endoscopic surgery. Exoscopy is similar to endoscopy in that both present an image produced by a scope onto a video monitor. The difference is that the camera is positioned outside the surgical field in exoscopy, allowing the surgeons to observe the surgical field of view with the naked eye [ 4]. Gidenberg and Labuz [ 2, 3] first reported use of an exoscope using the endoscopic system. The authors devised a video camera mounted on a stereotactic frame and that integrated the video image with a computergenerated 3D view to allow visualization of the virtual mass at the selected depth on the monitor in real time. Mamelak et al. [ 8] reported preliminary research regarding the HD exoscopic system for neurosurgery in an animal model. The authors used an 8-mm-diameter rigid lens telescope with 3-chip HD digital camera and HD monitor. The authors reported that image quality with the exoscopic system was similar to that with the OM even at high magnification, and the system was easy to manipulate and comfortable during surgery. The lack of stereopsis was a limitation but was compensated for with repeated procedures. The authors reported 16 clinical cases in the literature in which a 10-mm-diameter rigid-lens telescope with a focal distance of 20 cm was used, held by a pneumatic holder over the operative field. The authors reported the same results using an exoscopic system in which the image quality was nearly the same as with the OM, but lacking stereopsis. They also reported that positioning and focusing of exoscope system were relatively low compared to OM [ 9]. The main drawback of stereopsis in exoscopy was overcome by development of 3D exoscopes. Rossini et al. [ 13] reported preliminary experience in petrosal meningioma using VITOM 3D, a 3D technology combined with 4K resolution. The VITOM 3D showed advantages including ergonomics, versatility, and depth of field compared with the OM. However, the authors suggested that the holder arm and repositioning system, refocusing, magnification need to be ameliorated. Oertel and Burkhardt [ 11] reported the initial experience of using VITOM 3D in five cranial and 11 spinal surgeries and assessed instrument handling, intraoperative repositioning and handling, and comfort level, giving an excellent rating of 100%, with image quality equal to that of the OM. Beez et al. [ 1] reported their experience using VITOM 3D in pediatric neurosurgery. The authors answered questionnaires regarding ease of preparation, image definition, magnification, illumination, field of view, ergonomics, accessibility of the surgical field, and general user friendliness in three operations. Although the microscope was superior in magnification, field of view, illumination, and user friendliness, the authors reported the exoscope to be advantageous in ergonomics and accessibility to the surgical field. Ricciardi et al. [ 12] systemically reviewed 29 reports in the literature of exoscope use in neurosurgery on 574 patients. The authors concluded that the exoscope was largely considered superior or equivalent to the OM in ergonomic comfort, educational purposes, image quality, magnification, lighting, and cost, although some surgeons preferred the OM for better stereoscopic vision. This study was the first to analyze the experience of two 3D exoscopes compared to OM using statistical methods in brain tumor surgeries. Two exoscopes with different image resolutions, VOMS-100 with full HD and VITOM 3D with 4K, were used and compared with the OM, Kinevo 900. VOMS-100 was generally inferior to the OM in overall image quality including magnification and brightness. However, the VITOM 3D was equivalent to the OM in overall image quality and brightness, except for magnification. The different results obtained between exoscopes were potentially caused by different specifications such as the image resolution of monitor and camera. All 3D exoscopes showed advantages in accessibility of instruments in the surgical field due to the relatively long working distance and direct observation of the surgical field with the naked eye. A recently developed OM, Kinevo 900, also has a long working distance, but the bulky head of the OM can hinder direct view of the surgical field. The smaller amount of space occupied is also an advantage in the modern neurosurgical operating theater because more equipment such as that for neuronavigation, devices for intraoperative neurophysiologic monitoring, or ultrasonic aspirator are used simultaneously and require space. Ergonomics and educational usefulness were not different between exoscopes and the OM in the present study, which may be due to the use of the highly advanced OM, Kinevo 900. However, the exoscopes studied were limited in integration with neuronavigation and detection of fluorescence, which may be overcome in future models.
CONCLUSION
Although not yet commonly used in brain tumor surgery, the 3D exoscope has emerged as a new optical system alternative to OMs. The 3D exoscopes can provide comparable image quality and brightness if the appropriate resolution is offered. In addition, surgeons can view the surgical field with the naked eye and easily access instruments in the surgical field. Furthermore, the size of exoscopes is generally smaller and the cost is less than those of OMs. More advanced exoscopes most likely will be used as substitutes for OMs in the future.
Fig. 1.
Operating room during exoscopic surgery using VITOM 3D. The 3D monitor is displayed in front of surgeons. Surgeons can see the surgical field with the naked eye due to the long working distance of the exoscope and can watch the magnified image on the monitor while wearing 3D glasses. 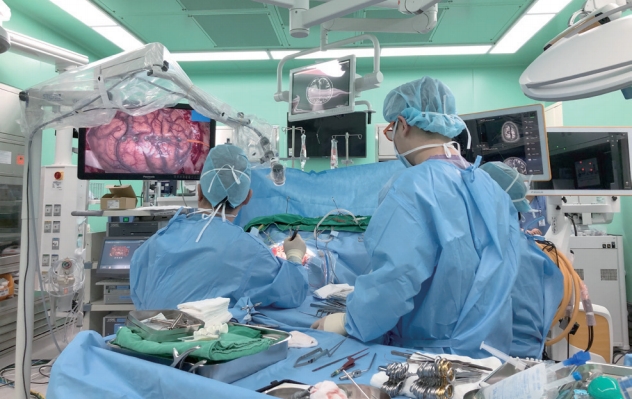
Fig. 2.
Results of questionnaire regarding the experience of using exoscopes (VOMS-100 or VITOM 3D) compared with the OM (Kinevo 900). A : Questions regarding image quality on the display monitor. VITOM 3D was equivalent to Kinevo 900 except for quality of the magnified image (Q. 3). However, VOMS-100 was inferior to Kinevo 900 in all aspects (Q. 1-5). B : Questions regarding handling of equipment. Exoscopes were inferior in focusing on the surgical field (Q. 7). However, exoscopes were superior in accessibility of instruments in the surgical field (Q. 6) and amount of space occupied in the operating theater (Q. 9). C : Questions regarding ergonomics and educational usefulness. Difference was not observed between exoscope and OM. D : Questions regarding 3D glasses and expectations. Discomfort in wearing the 3D glasses was not reported, and respondents had high expectations regarding substitution of exoscopes for the OM. *p<0.05. Q : question, N/C : no choice, OM : operating microscope. 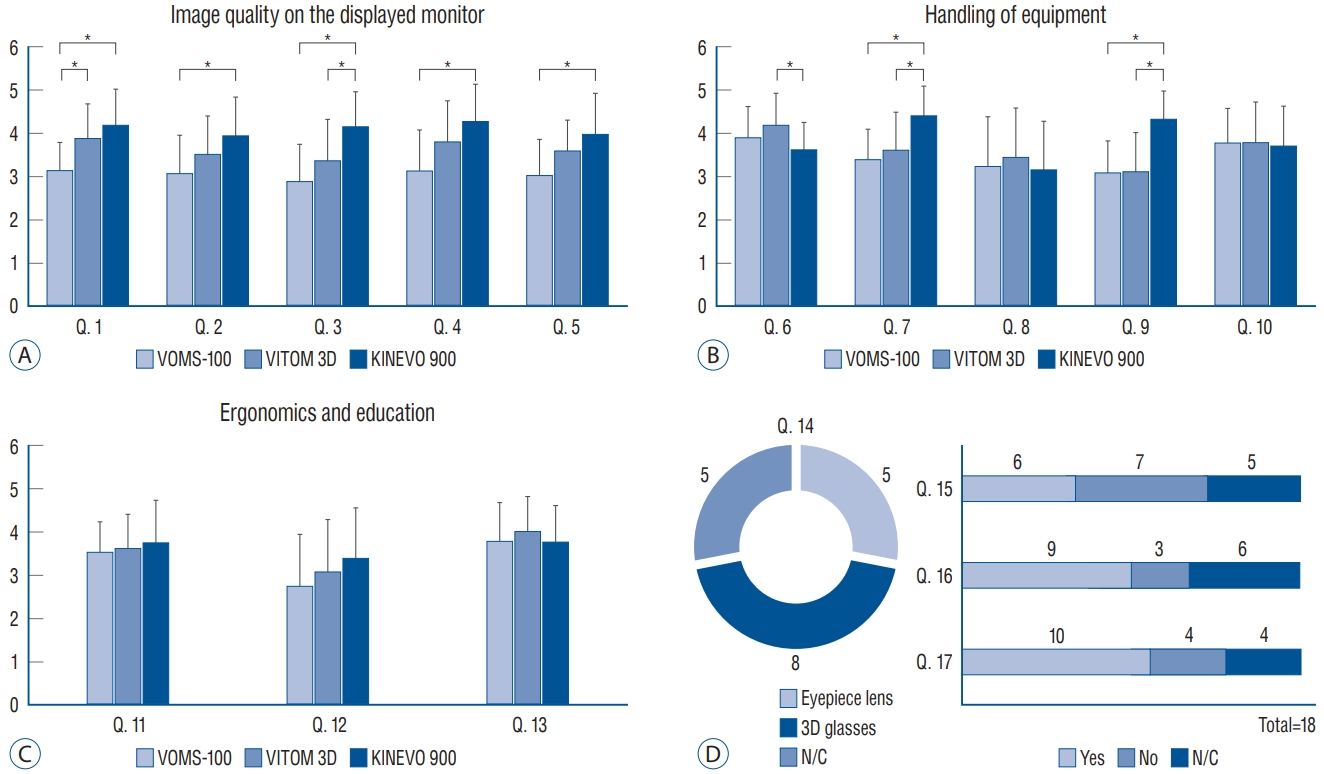
Table 1.
Clinical characteristics of patients performed the neurosurgical resection under exoscopes
|
No |
Age |
Sex |
Type of exoscope |
Histopathology |
Location |
Size (cm) |
Volume (mL) |
Extent of resection |
Surgical complication |
|
1 |
68 |
M |
VOMS-100 |
Metastasis |
Cbll |
3.2×3.6×2.5 |
15.6 |
GTR |
None |
|
2 |
78 |
F |
VOMS-100 |
GBM |
Parietal |
5.6×5.1×3.9 |
43.1 |
GTR |
None |
|
3 |
66 |
F |
VOMS-100 |
Metastasis |
Frontal |
4.3×3.3×4.0 |
27.9 |
GTR |
None |
|
4 |
67 |
M |
VOMS-100 |
Meningioma |
Frontal convexity |
4.8×3.8×5.6 |
48.1 |
GTR |
None |
|
5 |
66 |
F |
VOMS-100 |
Meningioma |
Parasagittal |
9×4.5×8.2 |
131 |
GTR |
None |
|
6 |
49 |
F |
VITOM 3D |
Metastasis |
Medial prefrontal |
4×3.4×3.8 |
19.3 |
STR |
None |
|
7 |
57 |
M |
VITOM 3D |
Anaplastic astrocytoma |
Frontal |
6.2×5.0×4.6 |
66.1 |
STR |
None |
|
8 |
39 |
F |
VITOM 3D |
Anaplastic xanthoastrocytoma |
Frontal |
8×5.6×4.8 |
65.3 |
GTR |
None |
|
9 |
62 |
M |
VITOM 3D |
GBM |
Medial prefrontal |
3.6×3.3×3.8 |
16.1 |
GTR |
None |
|
10 |
26 |
F |
VITOM 3D |
Meningioma |
Frontal convexity |
3.8×3×2.1 |
20.2 |
GTR |
None |
|
11 |
39 |
M |
VITOM 3D |
Hemangioblastoma |
Cbll |
4.3×3×2.6 |
11.8 |
GTR |
Remote EDH |
Table 2.
Comparison of technical specifications between exoscopes and microscope
|
VOMS-100 |
VITOM 3D |
Kinevo 900 |
|
Working distance (cm) |
21/22/23/31/35 |
20-50 |
20-62.5 |
|
Optical zoom |
1-2× |
1-4× |
1-6× |
|
Magnification |
7-80× |
8-30× |
0.9-41.3× |
|
Depth of field (focal length) (cm) |
5/9.5/15/25 |
3.5-10 |
17/26 |
|
Fluorescence detection |
No |
No |
Yes |
|
Integration with navigation system |
No |
No |
Yes |
|
Light source |
LED |
LED |
Xenon |
|
Auto focusing |
No |
No |
Yes |
|
Displayed video image |
Full-HD |
4K |
Full-HD/4K |
|
Auto-balance |
No |
No |
Yes |
|
Controller type |
Foot panel |
Mounted jog dial |
Foot panel |
|
Movement by controller |
Possible |
Impossible |
Possible |
|
Weight (kg) |
95 |
N/D |
395 |
|
Cost* (KRW) |
Approximately 50 million |
Approximately 150 million |
Approximately 500 million |
Table 3.
Questionnaire about the experience of exoscopes compared with microscope
|
Question |
VOMS-100 |
VITOM 3D |
KINEVO 900 |
|
Image quality on the displayed monitor |
|
|
1. How was the quality of the overall image? |
( )/5 |
( )/5 |
( )/5 |
|
2. How was the quality of the image when hemorrhage was visible in the surgical field? |
( )/5 |
( )/5 |
( )/5 |
|
3. How was the quality of the image when the surgical field was magnified? |
( )/5 |
( )/5 |
( )/5 |
|
4. How was the overall brightness of the image? |
( )/5 |
( )/5 |
( )/5 |
|
5. How was the brightness of the image when the deep surgical field was shown? |
( )/5 |
( )/5 |
( )/5 |
|
Handling of the equipment |
|
6. How easy was it to move instruments in the surgical field? |
( )/5 |
( )/5 |
( )/5 |
|
7. How easy was it to focus on the surgical field? |
( )/5 |
( )/5 |
( )/5 |
|
8. How convenient was it to prepare and install? |
( )/5 |
( )/5 |
( )/5 |
|
9. How much space did the equipment occupy in the operating theater? |
( )/5 |
( )/5 |
( )/5 |
|
10. How was the overall convenience during surgery? |
( )/5 |
( )/5 |
( )/5 |
|
Ergonomics |
|
11. How much eye fatigue did you experience during surgery? |
( )/5 |
( )/5 |
( )/5 |
|
12. How much body discomfort did you experience? |
( )/5 |
( )/5 |
( )/5 |
|
Educational usefulness |
|
13. How appropriate was it for educational purposes? |
( )/5 |
( )/5 |
( )/5 |
|
3D glasses |
|
14. Which was more comfortable, the eyepiece lenses or 3D glasses? |
(1) E. lens |
(2) 3D glasses |
(3) N/C |
|
15. Did you experience dizziness while wearing the 3D glasses? |
(1) Yes |
(2) No |
(3) N/C |
|
Expectation |
|
16. Do you think the exoscope can currently be used as a substitute for the OM in brain tumor surgery? |
(1) Yes |
(2) No |
(3) N/C |
|
17. Do you expect the exoscope will replace the OM in the future? |
(1) Yes |
(2) No |
(3) N/C |
References
1. Beez T, Munoz-Bendix C, Beseoglu K, Steiger HJ, Ahmadi SA : First clinical applications of a high-definition three-dimensional exoscope in pediatric neurosurgery. Cureus 10 : e2108, 2018    2. Gildenberg PL, Labuz J : Stereotactic craniotomy with the exoscope. Stereotact Funct Neurosurg 68 : 64-71, 1997   3. Gildenberg PL, Labuz J : Use of a volumetric target for image-guided surgery. Neurosurgery 59 : 651-659, 2006   4. Gildenberg PL, Ledoux R, Cosman E, Labuz J : The exoscope--a framebased video/graphics system for intraoperative guidance of surgical resection. Stereotact Funct Neurosurg 63 : 23-25, 1994   5. Jacobson JH 2nd, Wallman LJ, Schumacher GA, Flanagan M, Suarez EL, Donaghy RM : Microsurgery as an aid to middle cerebral artery endarterectomy. J Neurosurg 19 : 108-115, 1962   7. Kuchta J, Simons P : Spinal neurosurgery with the head-mounted “varioscope” microscope. Cent Eur Neurosurg 70 : 98-100, 2009   8. Mamelak AN, Danielpour M, Black KL, Hagike M, Berci G : A high-definition exoscope system for neurosurgery and other microsurgical disciplines: preliminary report. Surg Innov 15 : 38-46, 2008   9. Mamelak AN, Nobuto T, Berci G : Initial clinical experience with a highdefinition exoscope system for microneurosurgery. Neurosurgery 67 : 476-483, 2010   10. Nishiyama K : From exoscope into the next generation. J Korean Neurosurg Soc 60 : 289-293, 2017    11. Oertel JM, Burkhardt BW : Vitom-3D for exoscopic neurosurgery: initial experience in cranial and spinal procedures. World Neurosurg 105 : 153-162, 2017   12. Ricciardi L, Chaichana KL, Cardia A, Stifano V, Rossini Z, Olivi A, et al : The exoscope in neurosurgery: an innovative “point of view”. A systematic review of the technical, surgical, and educational aspects. World Neurosurg 124 : 136-144, 2019   13. Rossini Z, Cardia A, Milani D, Lasio GB, Fornari M, D’Angelo V : VITOM 3D: preliminary experience in cranial surgery. World Neurosurg 107 : 663-668, 2017   15. Uluç K, Kujoth GC, Başkaya MK : Operating microscopes: past, present, and future. Neurosurg Focus 27 : E4, 2009  
|
|


















