Bai, Zhou, Song, Ren, and Xiao: Drilling off the Petrosal Apex and Opening the Upper Wall of Meckel’s Cave Are the Key Elements of Good Outcomes in the Treatment of Trigeminal Neuralgia Secondary to Petrous Apex Meningioma
Abstract
Objective
The surgical management of trigeminal neuralgia (TN) caused by petrous apex meningioma (PAM) is still a challenge because of the lesion’s deep location and the surrounding complex structures. The authors describe the intradural anterior transpetrosal approach (ATPA) and its effect on the treatment of TN secondary to PAM.
Methods
A retrospective analysis of 15 patients with TN secondary to PAM who underwent surgery via the intradural ATPA was conducted. The key techniques, which included drilling off the petrosal apex (PA) and opening the upper wall of Meckel’s cave (MC), are described in detail.
Results
Total removal of the tumor and complete pain relief (Barrow Neurological Institute I) were achieved in all 15 patients without significant morbidity. Five patients developed new facial numbness postoperatively, which disappeared within three months after surgery. The postoperative magnetic resonance imaging showed temporal lobe swelling in three patients, but no clinical symptoms. One patient had cerebrospinal fluid leakage and was managed with bed rest and temporary lumbar drainage. One patient had an intracranial infection and was treated with antibiotics. By the last follow up, no patients had pain relapse or/and tumor recurrence. It is worth noting that the vascular compression at the root of the trigeminal nerve was found in one patient during the operation.
Conclusion
Our experience suggests that drilling off the PA and opening the upper wall of the MC are key elements for a good outcome of the treatment of TN secondary to PAM. The intradural ATPA has the advantages for both tumor resection and pain relief.
Key Words: Anterior transpetrous approach · Microsurgery · Meningioma, petrous apex · Trigeminal nerve · Trigeminal neuralgia.
INTRODUCTION
Trigeminal neuralgia (TN) can result from structural compression from aberrant vessels and tumors, such as schwannomas, epidermoid cysts and meningiomas adjacent to the trigeminal ganglion, Meckel’s cave (MC) or brainstem [ 30, 39, 40]. There are many available methods to treat the TN caused by intracranial small tumor such as medical treatment, glycerol rhizotomy, percutaneous gasserian ganglion compression, radiofrequency trigeminal nerve ablation, alcohol injection and stereotactic radiosurgery (SRS). However, in the presence of persistent and recurrent TN, and growth of the tumor, microsurgery is an important management option for those patients [ 2, 6, 17]. Given the rarity of TN secondary to petrous apex meningioma (PAM), and the associated surgical challenges, there are few studies describing optimal management of this condition [ 11, 35]. The aim of this study was to describe the key technique of the intradural anterior transpetrous approach (ATPA) in detail and its advantages for TN relief and tumor control.
MATERIALS AND METHODS
This research was approved by the Institutional Review Board of Xuanwu Hospital, Capital Medical University.
Patient population
Totally 15 patients diagnosed as PAM with secondary TN were included in this study from January 2015 to October 2019. There were four male and 11 female patients, ranging in age between 42 and 67 years (mean, 51.4±6.6 years). The occurrences rate of the most common clinical symptoms and signs are listed in Table 1. Tumor size was measured as the greatest contrast-enhancing tumor diameter on magnetic resonance imaging (MRI) and graded according to the classification of Natarajan et al. [ 28] ( Table 2). The courses of TN ranged from 1 to 36 months (mean, 10.4±17.3 months). TN was graded according to the Barrow Neurological Institute (BNI) pain scale ( Table 3). The usage of carbamazepine before the surgery was collected in Table 4. Four patients were not able to tolerate the side effects of carbamazepine and did not take regular medication, while all the other 11 patients took the drug 0.4 g/d or 0.6g/d for more than 6 months. The intradural ATPA was performed in all 15 patients. Simpson grade I or II was described as gross total resection. MRI scans were performed within 3 days after operation to evaluate tumor resection degree ( Fig. 1) and at 3 months, 1 year and every year thereafter to assess tumor recurrence. None of the patients received radiosurgery or other treatments before the surgery. The follow-up was performed from 12 months to 53 months (mean, 33.6±13.2 months). All the serial follow-up information was obtained via direct contact with the patient. The improvements of clinical symptoms were recorded in Table 1.
Operative techniques
The intradural ATPA was performed in all 15 patients. All procedures were completed by the same senior neurosurgeon in four hospitals. The key techniques included drilling off the petrosal apex (PA) and opening the upper wall of MC were used in all patients regardless of tumor size and invasion into MC. Lumbar drainage was performed in patients with an elevated risk of venous congestion and increased intracranial pressure, including those with large tumors, comorbidities, obesity, and the elderly. All patients underwent neurophysiological monitoring during the surgery. The intradural ATPA was better described elsewhere [ 43], the key technique was detailed below.
Position, skin incision and craniotomy
The patient was placed in the lateral position, and a preauricular curving skin incision was made ( Fig. 2A). The skin incision began at the lower edge of the zygomatic arch, about 2 cm anterior to the tragal notch, and extended upward and then curving to about 4 cm above the top of the auricular ( Fig. 2B). The temporalis muscle was incised along the red dashed line ( Fig. 2C). A burr hole was made and a squamous temporal bone flap was elevated ( Fig. 2B and C). The craniotomy was above the external auditory meatus with one-third posterior and two-thirds anterior to the external auditory meatus ( Fig. 2B and C). Bone window was removed to the floor of the middle cranial fossa with ronguers and drill.
Drilling off the petrous apex bone
After dura was opened, cerebrospinal fluid (CSF) was released either by elevated the temporal lobe or through the lumbar drainage. The arcuate eminence (AE), PA and superior petrosal sinus (SPS) were identified ( Fig. 3B) and the dura on the PA was coagulated and incised along the SPS. The trigeminal impression, the posterior edge of petrous ridge and AE were used as the land markers during the drilling off the petrous apex bone ( Fig. 3A). The drilling began internally from the trigeminal impression and extended about 1.5 cm laterally, forwardly not exceeding 6 mm from the posterior edge of the petrous ridge, and not exceeding 8 mm in depth from the surface of the petrous bone ( Fig. 3C and D).
Tumor removal after opening MC and removing tentorium
After the PA bone was removed, the upper wall of MC was opened along the lateral margin to loosen the trigeminal nerve, and a part of the upper wall of MC was removed ( Fig. 4A) so that the tumor in MC can be removed easily in a straight view ( Fig. 4B). The SPS was elevated from petrosal bone and then cauterized and divided closely to the medial petrous bone to avoid injury to lateral vessels draining into the SPS. The part of the tentorium covering the posterior fossa and between trochlear nerve and trigeminal nerve was removed ( Fig. 4A), then the tumor base located in the petroclival area was dissected under direct view and the tumor was removed in a piecemeal fashion ( Fig. 4C).
Protection of the cranial nerves
Cranial nerves should be protected carefully during the tumor removal, especially when the tumor size was larger than 2.5 cm because that the trochlear nerve, abductor nerve and oculomotor nerve would be probably encased by tumor. Our surgical strategies to protect cranial nerves are as follows : 1) a small part of the tentorium covering the posterior fossa and between trochlear nerve and trigeminal nerve was removed so that the tumor base medial to trigeminal nerve in clivus area could be dissected firstly under direct view. 2) The tumor was enucleated as much as possible so as to loosen the cranial nerve surrounding the tumor. 3) The oculomotor nerve was identified at the inside of the tumor, and then separated following its course to the entrance of cavernous sinus. And 4) the abductor nerve was identified laterally near the brainstem, and separated from brain stem to the entrance of the Dorello's canal following its course.
The protection of temporal lobe and drainage vein and the prevention of CSF leakage
Brain tissue loose techniques, including preoperative lumbar drainage, use of mannitol, hyperventilation and head up tilt, have been used to reduce temporal lobe injury due to stretch. And covering gelatin sponge to the temporal lobe is also an effective protective measure. Sufficiently dissecting the drainage vein which passes through the dura matter of skull base can reduce the vein injury when the drainage vein is stretched ( Fig. 5). The air cells may be opened after drilling off the PA, which was possible to result in CSF leakage. If the air cells were not explicitly open, a piece of muscle with fibrin glue would be used to seal the PA after the tumor was totally removed ( Fig. 6). Otherwise, when the air cells were confirmed open, both the bone wax and the muscle were used to seal the air cells of PA to prevent the CSF leakage.
RESULTS
The gross total resection of the tumor was performed in all the 15 patients. According to BNI pain intensity scale there were four patients (26.7%) in grade III, 10 patients (66.7%) in grade IV, one patient (6.7%) in grade V and no patients in grade I or II before operation ( Table 3). Carbamazepine had been used in all the patients to control pain before operation, and was effective for eight patients (53.3%) while ineffective for three patients (20.0%) ( Table 4). Four patients (26.7%) were intolerable to carbamazepine because of its side effects such as dizziness, nausea and drowsiness ( Table 4). After surgery all the 15 patients had complete pain relief to grade I and no recurrence was found during the follow up period ( Table 3). The preoperative symptoms and signs were improved greatly ( Table 1). Five patients developed new facial numbness postoperatively, which disappeared in 3 months in all the cases without special treatment. The postoperative MRI showed temporal lobe swelling in three patients, but no clinical symptoms. One patient experienced CSF leakage and was managed with bed rest and temporary lumbar drainage. One patient had an intracranial infection and was treated with antibiotics. It is worth noting that vascular compression at the root of the trigeminal nerve was found in one patient during the operation.
DISCUSSION
Treatment of TN secondary to PAM
TN secondary to PAM is rarely reported, it represents up to 6% of all cases of TN [ 2]. The deep location and surrounding complex structures make surgical removal of PAM challenging. SRS is regarded as an alternative approach for tumor-related TN and is getting increasingly popular, in part due to the lower risks and cost compared to surgery [ 5, 19, 27, 29]. Tuleasca et al. [ 42] performed a systematic review showing initial freedom from pain (FFP) (BIN I and II) without medication ranged from 28.6% to 75% (mean, 53.1%; median, 52.1%) for Gamma Knife. However, the symptoms of TN can persist because the tumor does not disappear immediately or relapse in a delayed fashion due to tumor regrowth after SRS. In these instances, as well as for patients with large tumors, SRS is less effective than microsurgery for pain relief [ 3, 18, 35, 44]. Bir et al. [ 4] thought microsurgical resection is superior to Gamma Knife radiosurgery in achieving and maintaining pain-free status in patients with recurrent trigeminal pain associated with PM through reviewing six patients experienced recurrent TN. Microsurgery was not only widely used as the primary treatment for intracranial tumors, but also was used to control tumor-related TN with a relatively low complication rate in the past decades [ 13, 32, 34]. Hegazy et al. [ 15] selected microsurgery to treat 17 patients with small PAM and secondary refractory TN, and a complete FFP without medication was achieved in 14 patients (82.4%), with very low morbidity, no mortality, and 100% tumor control. Although taking carbamazepine is effective for controlling pain in early times for most patients, it would become ineffective and the pain would relapse as the tumor grows. Furthermore, some patients could not tolerate the side effects of the drug such as drowsiness, dizziness, etc. Other treatment options are also available including glycerol rhizotomy, percutaneous Gasserian ganglion compression, alcohol injection. However, the recurrence rate could reach up to 50% 24 months after treatment [ 22]. Therefore, even if the tumor is relatively small, we would advocate surgical treatment because of fewer difficulties and far lower risks of small tumor surgeries compared to those of the large ones.
Surgical approaches for PAM
Many factors affect the choice of surgical approach for PAM including the location, extent of tumor involvement, and experience of the surgeon. Due to the sufficient tumor exposing and short work distance, the presigmoid transpetrosal approach once was used widely [ 8, 26, 28, 38]. However, the long time and destructive craniotomy resulted in consequent increase complications including high risk of infection, hearing impairment, and high CSF leak risk which limited greatly the application of the approach in recent years [ 46]. The Suboccipital retrosigmoid approach is widely used to remove PCM [ 1, 7, 9, 10, 25, 36]. It is a simple, effective approach for the PCM and familiar to most neurosurgeons. However, it could not provide adequate exposure for those extended into MC or middle cranial fossa. To overcome this pitfall, Samii et al. [ 37] described a method, retrosigmoid suprameatal intradural approach, which could expand the surgical view into MC and posterior part of the cavernous sinus. However, the extended bone drilling in posterior petrosectomy adds longer operative times and a worrisome increase in morbidity when tumor extended to middle fossa [ 33]. Sub-temporal approach was also selected to remove PCM, but the exposure of the base of PCM is not adequate in this approach, and the exploration of the trigeminal root area could not be carried out because of the obstacle of PA bone [ 14, 23, 45]. Transzygomatic approach was selected as the only approach when tumor invade into the anterior loop of the internal carotid artery (ICA), cavernous sinus, and upper clivus [ 24], but it does not provide good exposure of the cerebellopontine angle area.
Intradural ATPA
The ATPA was initially described by Kawase et al. [ 21]. The key step is to remove the PA bone. The removal of PA bone could be done either by extradurally or by intadurally. The PA bone was drilled off extradurally in typical ATPA. The advantages of typical ATPA include less concerns about brain tissue contusion and clearer land markers such as greater superficial petrosal nerve (GSPN; lateral), SPS (medial), and AE. The disadvantages of typical ATPA include blood oozing when elevating the dura, relatively higher the risk of CSF leakage because of extensive dura dissection in the skull base, and facial paralysis caused by the retraction of GSPN during dissection [ 16, 41, 43]. An intradural ATPA as described elsewhere was selected in our cases and the PA bone was drilled off intradurally [ 43]. The main concern of intradural ATPA is that the GSPN, AE and PA cannot be seen due to the dura mater coverage. In order to overcome the drawback of intradural ATPA as described above, the trigeminal impression and the posterior ridge of petrosal bone were used as the land markers during the PA bone removal. Diaz Day [ 11] also found that bone removal at the petrous ridge, lateral to the trigeminal impression, is the most reliable starting point. The navigation can also be used to locate the PA accurately. The advantage of intradural bone drilling is that the range of drilling can be individualized. A limited PA bone removal could decrease the risk of injury to critical local structures such as ICA [ 9, 12]. Intradural ATPA also avoided excessive dissection of the dura at the base of the middle cranial fossa, which reduced both the incidence of facial paralysis result from the stretch of the GSPN and epidural bleeding compared to typical ATPA. Removal of PA bone could increase the mobility of trigeminal nerve which facilitate the tumor removal and functional preservation of trigeminal nerve. It could provide adequate exposure of the PCM base in clivus area and pons, and also provided exposure of the whole intracranial segment of trigeminal nerve, so that the exploration and the treatment of the vascular compression on trigeminal nerve could be performed.
CSF leakage is a complication that many neurosurgeons worry about. The main cause of CSF leakage is opening of air cells after the removal of petrous apex. Both bone wax and muscle with fibrin glue were used to seal the air cells. Among the patients we reported, one case developed CSF and cured after 2 weeks of bed rest and temporary lumbar drainage. There are two main causes of temporal lobe injury in ATPA, one is the mechanical stretch of the temporal lobe, the other is the damage of the temporal drainage vein. Temporal lobe injury can be reduced by loosening brain tissue and dissecting the vein from the dura mater of the skull base. In this group of cases, three developed temporal lobe mild swelling on MRI, but none of them showed clinical symptoms.
The location of tumor and removing upper wall of MC
PAM is a subtype of petroclival meningioma which is classified into four subtypes by Kawase et al. [ 20] according to the location of the major tumor attachment and displacement of the trigeminal nerve. The PA meningioma is where the tumor is attached to the petrous apex and lies lateral to the trigeminal nerve and medial to the internal auditory canal, with superomedial shift of the trigeminal nerve [ 20]. However, in our cases, 10 (66.7%) had tumors with a diameter of less than 2.5 cm, which strictly meet the definition of PAM. The other five (33.3%) had tumors with a diameter more than 2.5 cm, and part of the tumor exceeded the area described above. However, we still classify these tumors as PAM because the main body of them were still located on the petrous apex. There is agreement that the best treatment for tumor induced TN includes removal of the tumor and obtaining relief from neuralgic pain [ 31]. In some cases we studied, the tumor grows into the MC, and the upper wall of the MC was also a part of the tumor base. Therefore, the removal of a part of the upper wall of MC could reduce the tumor recurrence. Opening the MC could also provide a better exposure to the tumor in MC, so that a complete tumor resection and a better functional preservation of the trigeminal nerve could be achieved at the same time. After the tumor was removed, the trigeminal nerve was explored starting from the trigeminal root entry zone at the brainstem to the MC as described by others [ 12]. If there are vascular compression and arachnoid adhesions, they should be isolated and released. In our cases, vascular compression at the root of the trigeminal nerve was found only in one case and was isolated from the nerve root with Teflon during the operation. The limitations of this study are as follow : the number of retrospective cases is small, and the follow up period is relatively short. This conclusion still needs to be confirmed by recruitment of more patients and longer follow-up.
CONCLUSION
Intradural ATPA could provide adequate exposure of PA area from the trigeminal root entry zone at the brainstem to the MC, thus facilitate tumor removal, trigeminal nerve decompression and protection. Drilling off PA bone and opening the upper wall of the MC is helpful to remove the tumor in MC, decompress the branches of trigeminal nerve and preserve trigeminal nerve, thus are crucial for a good outcome after the surgical treatment of TN secondary to PAM. Both the use of brain tissue loose techniques and protection of drainage vein reduce the risk of temporal injury. Intradural ATPA is a safe and effective method for treatment of TN secondary to PAM.
Fig. 1.
Magnetic resonance imaging (MRI) images of petrous apex meningioma. A and B : Preoperative contrast MRI images. There is a tumor significant enhancement (white arrow) located at right petrous apex extended to MC and upper clivus. C and D : Postoperative contrast MRI images. The tumor was gross totally removed (white arrow). 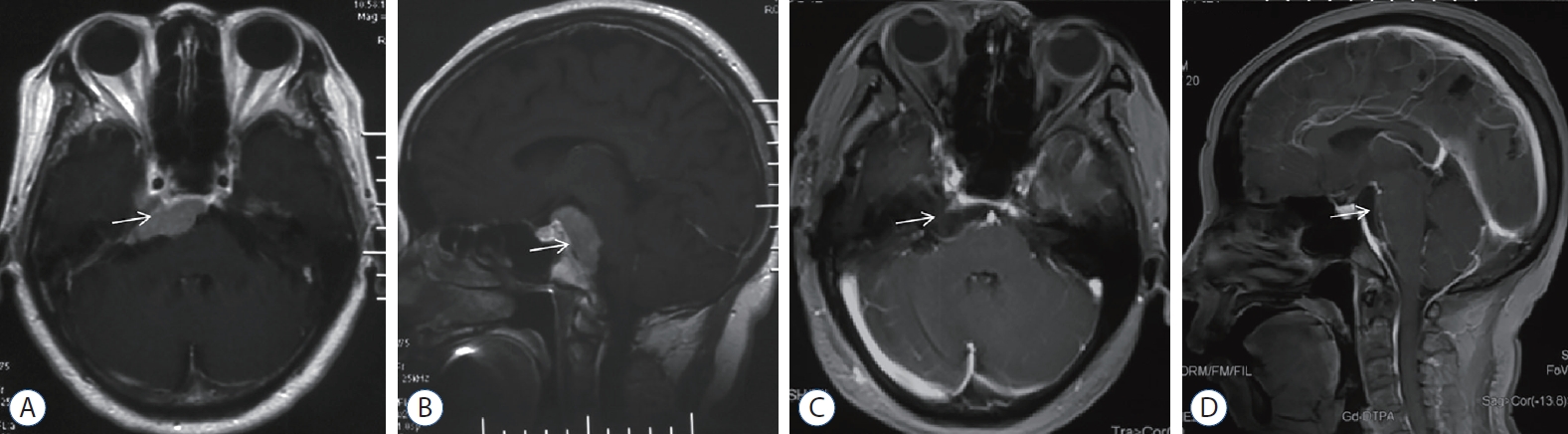
Fig. 2.
Position, skin incision and craniotomy of patient. A : The patient was placed in a lateral decubitus position. The head was fixed in a three-point Mayfield head holder. The curvilinear skin incision was used. B : Exposure of temporal bone. Burr hole (blue circle) was drilled above root of zygomatic arch (black thick arrow) and bone flap (black dashed line) was removed and the basement is at the level of the superior crest of the external auditory canal (black thin arrow). C : Sketch map showed burr hole (blue circle), bone flap (black dashed line) and incision of temporalis fascia and muscle (red dashed line). 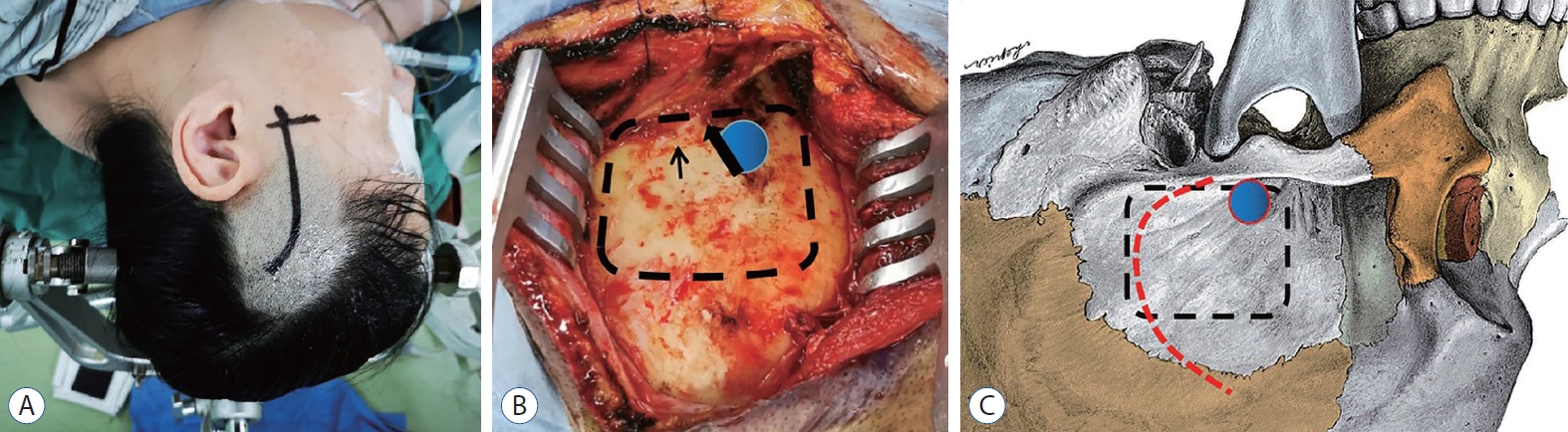
Fig. 3.
Removing bone of PA. A : Illustration showing the drilling extent of petrous apex bone. The trigeminal impression, the posterior edge of petrous ridge and AE were used as landmarks. B : PA, SPS, and AE was exposed by retracting temporal lobe in operation. C : Bone of PA (Kawase triangle) was drilled out (white dashed line) in operation. D : The postoperative thin-slice computed tomography shows the drilling range of petrous apex bone (white arrow). V : cranial nerve V, GSPN : great superficial petrous nerve, TT : tegmen tympani, VII : cranial nerve VII, VIII : VIII cranial nerve, SPS : superior petrosal sinus, PA : petrous apex, AE : arcuate eminence. 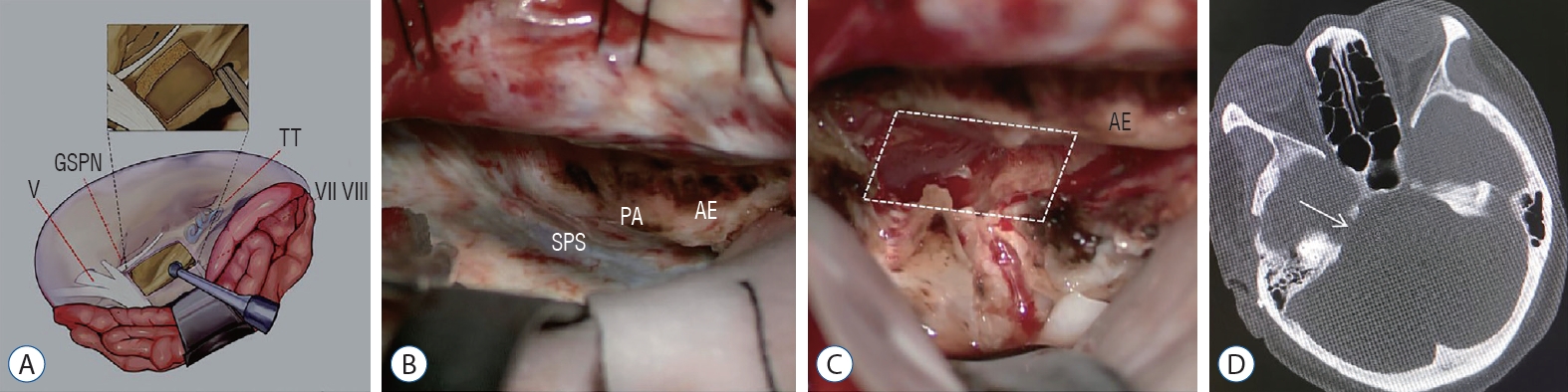
Fig. 4.
Opening the upper wall of the Meckel’s cave (MC) and removing tumor totally. A : Illustration showing the removing extent of the up wall of MC and the tentorium. B : Opening the Meckel Cave and removing the tumor in it during the operation. C : After removing tumor totally, trigeminal nerve, abducens nerve and BA were exposed. IV : cranial nerve, V : cranial nerve V, T : tumor, BA : basilar artery. 
Fig. 5.
The management of temporal drainage vein. A : The temporal drainage vein passed through the dura mater of skull base and drain posteriorly. B : The temporal drainage vein was dissected from dura mater of skull base and pull to temporal lobe. AE : arcuate eminence, Te : tentorium, TL : temporal lobe, V : vein. 
Fig. 6.
The management of air cells of PA after removing the tumor totally. A : The air cells may be opened after drilling off PA. B : The PA was sealed by a piece of muscle with fibrin glue. AE : arcuate eminence, M : muscle, PA : petrous apex. 
Table 1.
Improvement of preoperative symptoms and signs
|
Symptom/sign |
Preoperation (n=15) |
At the end of follow-up (n=15) |
|
Headache, dizziness |
5 (33.3) |
0 (0.0) |
|
Facial numbness |
6 (40.0) |
2 (13.3) |
|
Diplopia |
5 (33.3) |
1 (6.7) |
|
Limb weakness |
3 (20.0) |
0 (0.0) |
|
Visual deterioration |
1 (6.7) |
0 (0.0) |
Table 2.
|
Size category |
Number of patients (n=15) |
|
Small |
1 (6.7) |
|
Medium |
9 (60.0) |
|
Large |
5 (33.3) |
|
Giant |
0 (0.0) |
Table 3.
TN in preoperation and improvement in postoperation
|
BNI pain intensity scale |
Number of patients (n=15)
|
|
Preoperation |
Postoperation |
|
I |
0 (0.0) |
15 (100.0) |
|
II |
0 (0.0) |
0 (0.0) |
|
III |
4 (26.7) |
0 (0.0) |
|
IV |
10 (66.7) |
0 (0.0) |
|
V |
1 (6.7) |
0 (0.0) |
Table 4.
The usage of carbamazepine in preoperation
|
Usage of carbamazepine |
Number of patients (n=15) |
|
Effective |
8 (53.3) |
|
Ineffective |
3 (20.0) |
|
Intolerable (dizziness, nausea and drowsiness) |
4 (26.7) |
References
1. Almefty R, Dunn IF, Pravdenkova S, Abolfotoh M, Al-Mefty O : True petroclival meningiomas: results of surgical management. J Neurosurg 120 : 40-51, 2014   2. Barker FG 2nd, Jannetta PJ, Babu RP, Pomonis S, Bissonette DJ, Jho HD : Long-term outcome after operation for trigeminal neuralgia in patients with posterior fossa tumors. J Neurosurg 84 : 818-825, 1996   3. Berti A, Granville M, Wu X, Huang D, Schwade JG, Jacobson RE : Delayed development of trigeminal neuralgia after radiosurgical treatment of a tentorial meningioma. Cureus 9 : e16282017    4. Bir SC, Maiti TK, Bollam P, Nanda A : Management of recurrent trigeminal neuralgia associated with petroclival meningioma. J Neurol Surg B Skull Base 77 : 47-53, 2016   5. Black PM : Hormones, radiosurgery and virtual reality: new aspects of meningioma management. Can J Neurol Sci 24 : 302-306, 1997   6. Bullitt E, Tew JM, Boyd J : Intracranial tumors in patients with facial pain. J Neurosurg 64 : 865-871, 1986   7. Chen LF, Yu XG, Bu B, Xu BN, Zhou DB : The retrosigmoid approach to petroclival meningioma surgery. J Clin Neurosci 18 : 1656-1661, 2011   8. Cho CW, Al-Mefty O : Combined petrosal approach to petroclival meningiomas. Neurosurgery 51 : 708-716; discussion 716-718, 2002   9. Cui H, Zhou CF, Bao YH, Wang MS, Wang Y : Extended suboccipital retrosigmoid surgical approach is effective for resection of petrous apex meningioma. J Craniofac Surg 27 : e429-e433, 2016   10. de Divitiis O, Elefante A, de Divitiis E : Which strategy for petroclival tumors? World Neurosurg 86 : 33-35, 2016   11. Diaz Day J : The middle fossa approach and extended middle fossa approach: technique and operative nuances. Neurosurgery 70( 2 Suppl Operative):192-201, 2012   12. Dumot C, Brinzeu A, Berthiller J, Sindou M : Trigeminal neuralgia due to venous neurovascular conflicts: outcome after microvascular decompression in a series of 55 consecutive patients. Acta Neurochir (Wien) 159 : 237-249, 2017   13. Gerganov VM, Giordano M, Elolf E, Osamah A, Amir S, Madjid S : Operative management of patients with radiosurgery-related trigeminal neuralgia: analysis of the surgical morbidity and pain outcome. Clin Neurol Neurosurg 122 : 23-28, 2014   14. Goel A : Extended lateral subtemporal approach for petroclival meningiomas: report of experience with 24 cases. Br J Neurosurg 13 : 270-275, 1999   15. Hegazy A, Alfiki A, Adel MF, Alsawy MF, Al-Dash MF, Zein M, et al : Role of surgery for small petrous apex meningiomas causing refractory trigeminal neuropathy in the minimally invasive era. Neurol India 64 : 973-979, 2016   16. Ichimura S, Hori S, Hecht N, Czabanka M, Vajkoczy P : Intradural anterior transpetrosal approach. Neurosurg Rev 39 : 625-631, 2016   17. Kai M, Yongjie L : Clinical features and surgical management of cerebellopontine angle cholesteatoma that presented as trigeminal neuralgia. World Neurosurg 115 : e7-e12, 2018   18. Kano H, Awan NR, Flannery TJ, Iyer A, Flickinger JC, Lunsford LD, et al : Stereotactic radiosurgery for patients with trigeminal neuralgia associated with petroclival meningiomas. Stereotact Funct Neurosurg 89 : 17-24, 2011   19. Karam SD, Tai A, Wooster M, Rashid A, Chen R, Baig N, et al : Trigeminal neuralgia treatment outcomes following Gamma Knife radiosurgery with a minimum 3-year follow-up. J Radiat Oncol 3 : 125-130, 2014   20. Kawase T, Shiobara R, Ohira T, Toya S : Developmental patterns and characteristic symptoms of petroclival meningiomas. Neurol Med Chir (Tokyo) 36 : 1-6, 1996   21. Kawase T, Shiobara R, Toya S : Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery 28 : 869-875; discussion 875-876, 1991   22. Kouzounias K, Lind G, Schechtmann G, Winter J, Linderoth B : Comparison of percutaneous balloon compression and glycerol rhizotomy for the treatment of trigeminal neuralgia. J Neurosurg 113 : 486-492, 2010   23. Liao CH, Wang JT, Lin CF, Chen SC, Lin CJ, Hsu SPC, et al : Pretemporal trans-Meckel's cave transtentorial approach for large petroclival meningiomas. Neurosurg Focus 44 : E10, 2018  24. Little AS, Jittapiromsak P, Crawford NR, Deshmukh P, Preul MC, Spetzler RF, et al : Quantitative analysis of exposure of staged orbitozygomatic and retrosigmoid craniotomies for lesions of the clivus with supratentorial extension. Neurosurgery 62( 5 Suppl 2):ONS318-ONS323; discussion ONS323-ONS324, 2018  25. Martínez-Pérez R, Silveira-Bertazzo G, Rangel GG, Albiña P, Hardesty D, Carrau RL, et al : The historical perspective in approaches to the spheno-petro-clival meningiomas. Neurosurg Rev 44 : 51-60, 2021   26. Mathiesen T, Gerlich A, Kihlström L, Svensson M, Bagger-Sjöbäck D : Effects of using combined transpetrosal surgical approaches to treat petroclival meningiomas. Neurosurgery 60 : 982-991; discussion 991-992, 2007   27. Mureb MC, Dastazirgada Y, Benjamin C, Golfinos JG, Kondziolka D : Simultaneous treatment of petroclival meningiomas and the trigeminal nerve with gamma knife radiosurgery for tumor-related trigeminal neuralgia. World Neurosurg 139 : 242-244, 2020   28. Natarajan SK, Sekhar LN, Schessel D, Morita A : Petroclival meningiomas: multimodality treatment and outcomes at long-term follow-up. Neurosurgery 60 : 965-979; discussion 979-981, 2007   29. Nicolato A, Ferraresi P, Foroni R, Pasqualin A, Piovan E, Severi F, et al : Gamma knife radiosurgery in skull base meningiomas. Preliminary experience with 50 cases. Stereotact Funct Neurosurg 66 Suppl 1 : 112-120, 1996
30. Obermann M : Treatment options in trigeminal neuralgia. Ther Adv Neurol Disord 3 : 107-115, 2010    31. Pollock BE, Iuliano BA, Foote RL, Gorman DA : Stereotactic radiosurgery for tumor-related trigeminal pain. Neurosurgery 46 : 576-582; discussion 582-583, 2000   32. Ramina R, Neto MC, Fernandes YB, Silva EB, Mattei TA, Aguiar PH : Surgical removal of small petroclival meningiomas. Acta Neurochir (Wien) 150 : 431-438; discussion 438-439, 2008   33. Rolston JD, Han SJ, Lau CY, Berger MS, Parsa AT : Frequency and predictors of complications in neurological surgery: national trends from 2006 to 2011. J Neurosurg 120 : 736-745, 2014   34. Samii M, Carvalho GA, Tatagiba M, Matthies C : Surgical management of meningiomas originating in Meckel's cave. Neurosurgery 41 : 767-774; discussion 774-775, 1997   35. Samii M, Rosahl SK, Tatagiba MS : Microsurgical removal of a petrous apex meningioma after stereotactic radiation: technical case report. Neurosurgery 49 : 216-219; discussion 219-220, 2001   36. Samii M, Tatagiba M, Carvalho GA : Resection of large petroclival meningiomas by the simple retrosigmoid route. J Clin Neurosci 6 : 27-30, 1999   37. Samii M, Tatagiba M, Carvalho GA : Retrosigmoid intradural suprameatal approach to Meckel's cave and the middle fossa: surgical technique and outcome. J Neurosurg 92 : 235-241, 2000   38. Seifert V, Raabe A, Zimmermann M : Conservative (labyrinth-preserving) transpetrosal approach to the clivus and petroclival region--indications, complications, results and lessons learned. Acta Neurochir (Wien) 145 : 631-642; discussion 642, 2003   39. Shulev Y, Trashin A, Gordienko K : Secondary trigeminal neuralgia in cerebellopontine angle tumors. Skull Base 21 : 287-294, 2011    40. Squire SE, Chan MD, Furr RM, Lowell DA, Tatter SB, Ellis TL, et al : Gamma knife radiosurgery in the treatment of tumor-related facial pain. Stereotact Funct Neurosurg 90 : 145-150, 2012   41. Steiger HJ, Hänggi D, Stummer W, Winkler PA : Custom-tailored transdural anterior transpetrosal approach to ventral pons and retroclival regions. J Neurosurg 104 : 38-46, 2006   42. Tuleasca C, Régis J, Sahgal A, De Salles A, Hayashi M, Ma L, et al : Stereotactic radiosurgery for trigeminal neuralgia: a systematic review. J Neurosurg 130 : 733-757, 2018   43. Xiao X, Zhang L, Wu Z, Zhang J, Jia G, Tang J, et al : Surgical resection of large and giant petroclival meningiomas via a modified anterior transpetrous approach. Neurosurg Rev 36 : 587-593; discussion 593-594, 2013    44. Yamakami I, Higuchi Y, Horiguchi K, Saeki N : Treatment policy for petroclival meningioma based on tumor size: aiming radical removal in small tumors for obtaining cure without morbidity. Neurosurg Rev 34 : 327-334; discussion 334-335, 2011   45. Yang J, Liu YH, Ma SC, Wei L, Lin RS, Qi JF, et al : Subtemporal transtentorial petrosalapex approach for giant petroclival meningiomas: analyzation and evaluation of the clinical application. J Neurol Surg B Skull Base 73 : 54-63, 2012    46. Yang J, Ma SC, Fang T, Qi JF, Hu YS, Yu CJ : Subtemporal transpetrosal apex approach: study on its use in large and giant petroclival meningiomas. Chin Med J (Engl) 124 : 49-55, 2011 
|
|






















