Cho, Kim, Kim, Kim, Kang, and Lee: Endoscopic Fluorescence Angiography with Indocyanine Green : A Preclinical Study in the Swine
Abstract
Objective
Microscopic indocyanine green (ICG) angiography is useful for identifying the completeness of aneurysm clipping and the preservation of parent arteries and small perforators. Neuroendoscopy is helpful for visualizing structures beyond the straight line of the microscopic view. We evaluated our prototype of endoscopic ICG fluorescence angiography in swine, which we developed in order to combine the merits of microscopic ICG angiography and endoscopy.
Methods
Our endoscopic ICG system consists of a camera, a light source, a display and software. This system can simultaneously display real-time visible and near infrared fluorescence imaging on the same monitor. A commercially available endoscope was used, which was 4 mm in diameter and had an angle of 30┬░. A male crossbred swine was used.
Results
Under general anesthesia, a small craniotomy was performed and the brain surface of the swine was exposed. ICG was injected via the ear vein with a bolus dose of 0.3 mg/kg. Visible and ICG fluorescence images of cortical vessels were simultaneously observed on the display monitor at high resolution. The real-time merging of the visible and fluorescent images corresponded well.
Conclusion
Simultaneous visible color and ICG fluorescent imaging of the cortical vessels in the swine brain was satisfactory. Technical improvement and clinical implication are expected.
Key Words: Indocyanine green fluorescence angiography ┬Ę Aneurysm surgery ┬Ę Endoscopy.
INTRODUCTION
As the number of patients with unruptured cerebral aneurysms has increased, more attention has been given to the primary prevention of the aneurysm rupture 4). Endovascular intervention, one of the major treatment modalities, has begun to catch up with and surpass the surgery in the number of cases treated, based on the favorable outcomes and reduced morbidity/mortality 2,1220). In addition, recently, patients have shown a preference for endovascular intervention over surgery because of the cosmetic and psychological aspects. Scalpel surgeons are being asked to enhance their competitiveness and develop novel surgical devices and skills, such as minimally invasive keyhole surgery 8,1519), neuroendoscopic systems 7), indocyanine green (ICG) fluorescence angiography 5,18), intraoperative neurophysiological monitoring 16), and intraoperative cerebral angiography 1). Microscopic ICG fluorescence angiography is very helpful for detecting and readjusting unexpected arterial occlusion and incomplete clipping of cerebral aneurysms. However, the usefulness of ICG fluorescence angiography is limited when the microscopic view is poorly visualized or when ICG cannot absorb sufficient light to emit fluorescence. Therefore, these limitations can be overcome using the endoscopic systems that provide sufficient light illumination and excellent visualization of the operative fields. Thus, ICG fluorescence angiography would be very useful to supplement the shortcomings of microscopic ICG fluorescence. To date, there are 3 clinical reports regarding endoscopic ICG fluorescence angiography from Japan and Europe 5,1114). We independently developed an endoscopic camera system for ICG fluorescence angiography that can show the real-time simultaneous visible and fluorescent imaging. Using this system, we performed a preclinical study in swine.
MATERIALS AND METHODS
Animal study
All procedures were approved by the local Institutional Animal Care and Use Committee and were conducted according to international guidelines. A male crossbred swine (Landrace├ŚYorkshire├ŚDuroc), aged 3 months and with a weight of 38.5 kg, was used in this study. The swine was acclimated for one week before surgery. The procedures were conducted under general anesthesia. Anesthesia was induced intramuscularly with 5 mg/kg of zoletil and 2 mg/kg of xylazine. The swine was intubated and intravenous 0.9% normal saline was administered via the ear vein at a rate of 5 mL/kg/hr. Anesthesia was maintained with 2% to 4% isoflurane at a rate of 2-3 L/min and oxygen/nitrogen at a rate of 3 L/min. Vital parameters, such as arterial blood pressure, heart rate, and carbon dioxide levels, were continuously recorded. Expired carbon dioxide levels were maintained at 35-40 mm Hg. After the procedure, the animal was euthanized.
A 1├Ś2 cm small craniotomy was performed at a distance of 2 cm from the midline and 3 cm anterior to the bregma. The dura was incised and the frontal cortex was exposed. The ICG dye was injected into an ear vein at a dose of 0.3 mg/kg (25 mg dissolved in 5 mL of sterile normal saline).
Endoscopic ICG system
The endoscopic ICG system consists of an endoscope, a light source, a camera, a display, and a computer with software ( Fig. 1). Although all types of endoscopes with the same size of optical adaptor are adjustable, an endoscope 4 mm in diameter and 30┬░ in angle (MGB Endoskopische Gerate GmbH Berlin, Berlin, Germany) was used in this study. The light source is composed of a white light source and a near infrared (NIR) laser source ( Fig. 2). We developed a white light source that is installed with a HXP 120W/45C VIS mercury lamp (OSRAM GmbH, Munich, Germany). We also developed a NIR laser source that is installed with an 805 nm wavelength laser diode (RealLight, Beijing, China; FWHM <2 nm). ICG as a fluorophore binds to the hydrophobic core of human serum proteins, which shifts the absorption and emission spectra of ICG. After intravenous injection of ICG, the main peak of the absorption spectrum of ICG moves to 805 nm, so we chose an 805 nm laser that has the same wavelength as the ICG absorption peak 6). To deliver the combined light from the light source to the endoscope, we selected a 3 mm liquid light guide (Newport Corporation, Irvine, CA, USA). To simultaneously obtain the visible and ICG fluorescence images, we used a fluorescence navigation surgery (FNS) camera developed by Korea Electrotechnology Research Institute (KERI) ( Fig. 3). This camera has two charge-coupled device (CCD) image sensors : one is for visible color imaging, and the other is for NIR fluorescence imaging. A cube beam splitter was installed to divide the visible color image and the NIR fluorescence image. The reflected visible color image passes through an infrared (IR) cut-off filter to the color-image sensor and the penetrated NIR image goes to the mono-image sensor. The two images are processed on an image capture board and the data transfer board delivers the real time imaging to the computer by USB 3.0 interface. We used a 16-34 mm zoom optical adaptor (MGB Endoskopische Gerate GmbH Berlin, Berlin, Germany) to make the optical connection between the endoscope and the camera. The image monitoring program was developed by KERI and can show and record single or simultaneous images of visible light and fluorescence and a visible/fluorescence overlay image.
RESULTS
ICG fluorescence began to appear on the cerebral cortex with endoscopy in approximately 25 seconds after ICG dye injection, it became peak in 1 minute, then it became pale in approximately 5 minutes. Visible color and ICG fluorescent endoscopic images of cortical vessels were simultaneously observed on the display monitor with a high resolution. In addition, real-time merging images of the fluorescent cortical vessels could be made and marked in a purple color. The real-time merging of the visible color and fluorescent images corresponded well ( Fig. 4).
DISCUSSION
Since Raabe et al. 17) introduced microscopic ICG fluorescence angiography for cerebrovascular surgery in 2003, it has become a ubiquitous piece of equipment for aneurysm surgery as well as micro-anastomosis and surgery for arteriovenous fistulas and malformations. During aneurysm surgery, real-time identification of the blood flow in the vasculature within the operative field helps to improve surgical outcomes and reduce perioperative complications. This is because the repositioning and addition of clips can be promptly determined on the occlusion of parent arteries and perforators or incomplete clipping of the aneurysms. Intraoperative cerebral angiography is a good alternative 11). However, it is invasive and has a limitation to visualize the small perforators around the aneurysms in spite of the superiority in the acquisition of 3-dimensional imaging around the clipped aneurysms. In addition, supplementary facilities for intraoperative cerebral angiography are needed, and a time lag between the identification of compromise of the normal arteries and the repositioning of clips can result in an irreversible ischemic complication. On the other hand, microscopic ICG angiography also has some shortcomings : vascular structures beyond the line of microscopic view are hard to visualize and ICG fluorescence is weakly detected for the deeply located structures because of the limited spatial resolution and light illumination of microscopy. When keyhole surgery is performed, such limitations in microscopic ICG angiography become more profound. To overcome the drawbacks of microscopic ICG angiography, we decided to develop endoscopic ICG fluorescence angiography. In fact, endoscopic ICG angiography has been applied in other surgical fields before it was introduced in the field of neurosurgery, based on the property of ICG, which is circulated through the blood vessels, metabolized in the liver, and excreted via the bile duct into the intestines. Examples of the surgical applications of this technique include the detection of the sentinel lymph nodes of patients with gastric cancer 13), visualization of bleeding points in the gastrointestinal tract 9), assessment of tissue perfusion and bile tree in hepatopancreatobiliary surgery 22), and evaluation of free flap perfusion 3). Neuroendoscopy itself has been used mainly in skull base surgery via the transnasal approach and aneurysm surgery 7,1021). Meanwhile, the use of endoscopic ICG angiography in aneurysm surgery has recently been introduced 5,614). The authors consider endoscopic ICG angiography to be useful for confirming the occlusion of the aneurysms and the patency of blood flow in the parent arteries and perforators, which could not be visualized under the microscopy and its ICG angiography. Moreover, it is possible in the endoscopic ICG angiography to better magnify the areas of interest 5), obtain longer ICG fluorescence duration, and be more useful in the ruptured aneurysms 11), compared to the microscopic system. Light illumination from microscopy alone is reported to disturb the detection of ICG fluorescence with endoscopy 5). The advantage of our endoscopic ICG angiography system is that ICG fluorescence and visible color imaging can be simultaneously visualized and merged in a real-time manner. Previous microscopic or endoscopic ICG angiography systems cannot help but to demonstrate the visible color or NIR fluorescence imaging one at a time such that surgeons may feel some difficulty in comparing the real structures with fluorescence images during the surgery. However, the size of our prototype camera is larger than the previous one, which is an improvement.
We have further plans to improve this endoscopic ICG angiography technique. First, an endoscope with smaller diameter and flexibility may be more useful because the operative space is very narrow without retracting brain tissue or drilling bony structures. As the diameter becomes smaller, imaging resolution and transmission of the light illumination and emitting fluorescence tend to be diminished. Such technical considerations should be made. Second, distinct from the endonasal approach, which is performed through the bony nasal cavity, endoscopy with ICG angiography during aneurysm surgery reaches and stays around the targeted vascular structures intradurally adjacent to the brain under microscopic inspection. Separate displays for microscopy and endoscopy may be detrimental and time-consuming because endoscopy can damage the adjacent cerebrovascular structures. A unified display system that can visualize both the microscopic and endoscopic imaging in one piece may be useful in terms of safety and convenience.
CONCLUSION
Our endoscopic ICG fluorescence angiographic system showed satisfactory imaging resolution in a swine study. Different from the previous systems, it was possible to simultaneously display visible color and ICG fluorescent images, and make a real-time merging images of them. Technical supplementation and approval for the clinical implication are expected to support the application of aneurysm surgery as well as surgery for other cerebrovascular diseases.
Acknowledgements
This study was supported by a grant from Seoul Metropolitan Government and its Seoul Development Institute WR100001 for Russia Science Seoul in the frameworks of the Program for International Joint Research "Inviting & Supporting Project of Global Leading Institutions", as a part of the Seoul Research & Business Development Support Program.
References
1. Alexander TD, Macdonald RL, Weir B, Kowalczuk A : Intraoperative angiography in cerebral aneurysm surgery : a prospective study of 100 craniotomies. Neurosurgery 1996, 39 : 10-17, discussion 17-18   2. Andaluz N, Zuccarello M : Recent trends in the treatment of cerebral aneurysms : analysis of a nationwide inpatient database. J Neurosurg 2008, 108 : 1163-1169,   3. Betz CS, Zhorzel S, Schachenmayr H, Stepp H, Havel M, Siedek V, et al : Endoscopic measurements of free-flap perfusion in the head and neck region using red-excited Indocyanine Green : preliminary results. J Plast Reconstr Aesthet Surg 2009, 62 : 1602-1608,   4. Brown RD Jr, Broderick JP : Unruptured intracranial aneurysms : epidemiology, natural history, management options, and familial screening. Lancet Neurol 2014, 13 : 393-404,   5. Bruneau M, Appelboom G, Rynkowski M, Van Cutsem N, Mine B, De Witte O : Endoscope-integrated ICG technology : first application during intracranial aneurysm surgery. Neurosurg Rev 2013, 36 : 77-84, discussion 84-85   6. Ciamberlini C, Guarnieri V, Longobardi G, Poggi P, Donati MC, Panzardi G : Indocyanine green videoangiography using cooled charge-coupled devices in central serous choroidopathy. J Biomed Opt 1997, 2 : 218-225,   7. Fischer G, Oertel J, Perneczky A : Endoscopy in aneurysm surgery. Neurosurgery 2012, 70( 2 Suppl Operative):184-190, discussion 190-191   8. Hernesniemi J, Ishii K, Niemel├ż M, Smrcka M, Kivipelto L, Fujiki M, et al : Lateral supraorbital approach as an alternative to the classical pterional approach. Acta Neurochir Suppl 2005, 94 : 17-21,   9. Ishihara R, Iishi H, Kidu T, Yamamoto S, Miyoshi R, Inoue T, et al : Infrared endoscopic system for bleeding-point detection after flushing with indocyanine green solution (with videos). Gastrointest Endosc 2008, 68 : 975-981,   10. Litvack ZN, Zada G, Laws ER Jr : Indocyanine green fluorescence endoscopy for visual differentiation of pituitary tumor from surrounding structures. J Neurosurg 2012, 116 : 935-941,   11. Mielke D, Malinova V, Rohde V : Comparison of intraoperative microscopic and endoscopic ICG angiography in aneurysm surgery. Neurosurgery 2014, 10( Suppl 3):418-425, discussion 425   12. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al : : International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms : a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005, 366 : 809-817,   13. Nimura H, Narimiya N, Mitsumori N, Yamazaki Y, Yanaga K, Urashima M : Infrared ray electronic endoscopy combined with indocyanine green injection for detection of sentinel nodes of patients with gastric cancer. Br J Surg 2004, 91 : 575-579,   14. Nishiyama Y, Kinouchi H, Senbokuya N, Kato T, Kanemaru K, Yoshioka H, et al : Endoscopic indocyanine green video angiography in aneurysm surgery : an innovative method for intraoperative assessment of blood flow in vasculature hidden from microscopic view. J Neurosurg 2012, 117 : 302-308,   15. Park HS, Park SK, Han YM : Microsurgical experience with supraorbital keyhole operations on anterior circulation aneurysms. J Korean Neurosurg Soc 2009, 46 : 103-108,    16. Qui├▒ones-Hinojosa A, Alam M, Lyon R, Yingling CD, Lawton MT : Transcranial motor evoked potentials during basilar artery aneurysm surgery : technique application for 30 consecutive patients. Neurosurgery 2004, 54 : 916-924, discussion 924   17. Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V : Near-infrared indocyanine green video angiography : a new method for intraoperative assessment of vascular flow. Neurosurgery 2003, 52 : 132-139, discussion 139   18. Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, et al : Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg 2005, 103 : 982-989,   19. Reisch R, Perneczky A : Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery 2005, 57( 4 Suppl):242-255, discussion 242-255   20. Smith GA, Dagostino P, Maltenfort MG, Dumont AS, Ratliff JK : Geographic variation and regional trends in adoption of endovascular techniques for cerebral aneurysms. J Neurosurg 2011, 114 : 1768-1777,   21. Snyderman CH, Carrau RL, Kassam AB, Zanation A, Prevedello D, Gardner P, et al : Endoscopic skull base surgery : principles of endonasal oncological surgery. J Surg Oncol 2008, 97 : 658-664,   22. Verbeek FP, van der Vorst JR, Schaafsma BE, Hutteman M, Bonsing BA, van Leeuwen FW, et al : Image-guided hepatopancreatobiliary surgery using near-infrared fluorescent light. J Hepatobiliary Pancreat Sci 2012, 19 : 626-637,   
Fig.┬Ā1
Schematic illustration (A) and real model (B) of the endoscopic indocyanine green (ICG) fluorescence angiography.
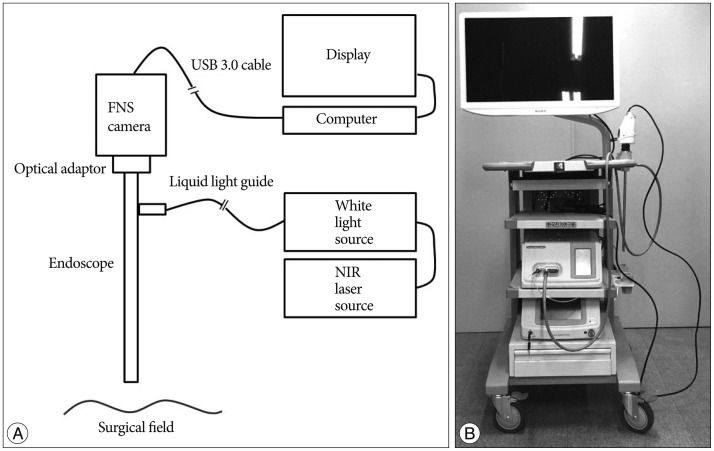
Fig.┬Ā2
Schematic illustration (A) and real model (B) of the white light and near infrared (NIR) laser sources.
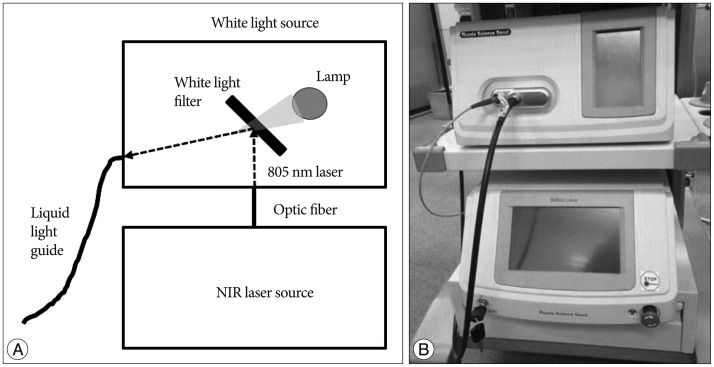
Fig.┬Ā3
Schematic illustration (A) and real model (B) of the fluorescence navigation surgery (FNS) camera.

Fig.┬Ā4
Endoscopic visible color and ICG fluorescent images. A : Photography of the exposed brain cortex in the swine. Endoscopic imaging was targeted for the dotted circle area. B : ICG fluorescent image (left), visible color image (middle), and and real-time merging image of ICG fluorescent and visible color images (right).

|
|



















