Yang, Kim, Lee, Jeong, Kwon, and Kim: Role of Adjunctive Tranexamic Acid in Facilitating Resolution of Chronic Subdural Hematoma after Surgery
Abstract
Objective
Chronic subdural hematoma (CSDH) is a common neurosurgical disease and generally treated with burr-hole surgery alone. Tranexamic acid (TXA) is an antifibrinolytic agent that potentially reduces recurrence rates and the residual hematoma volume. However, the role of postoperative TXA medication remains unclear to date. This study aimed to verify the effectiveness of adjunctive TXA in the view of early hematoma resolution.
Methods
Between January 2018 and September 2021, patients with CSDH who underwent burr-hole trephination in a single tertiary institute were reviewed. The study population was divided into three groups, TXA, non-TXA, and antithrombotics (AT) groups, according to the medical history of cardio-cerebrovascular disease and TXA administration. The primary endpoint was CSDH recurrence, defined as re-appearance or re-accumulation of CSDH requiring neurosurgical interventions. The secondary outcome was CSDH resolution, defined as complete or near-complete resorption of the CSDH. The CSDH resolution time and serial changes of hematoma thickness were also investigated.
Results
A total of 240 patients was included in the analysis consisting of 185 male and 55 female, with a median age of 74 years. During the median imaging follow-up period of 75 days, 222 patients were reached to the primary or secondary endpoint. TXA was administered as an adjunctive therapy in 41 patients (TXA group, 16.9%) while 114 patients were included in the non-TXA group (47.9%) and 85 were in the AT group. The recurrence rate was the lowest in the TXA group (2.4%), followed by non-TXA (7.0%) and AT (8.2%) groups. However, there was no statistical significance due to the small number of patients with recurrence. CSDH resolution was achieved in 206 patients, and the median estimated time to resolution was significantly faster in the TXA group (p<0.001). Adjunctive TXA administration was a significant positive factor for achieving CSDH resolution (p<0.001). The hematoma thickness was comparable among the three groups at the initial time and after surgery. However, CSDH thickness in the TXA group decreased abruptly in a month and showed a significant difference from that in the other groups (p<0.001). There was no TXA-related adverse event.
Conclusion
The adjunctive use of TXA after CSDH surgery significantly facilitated the resorption of residual CSDH and resulted in the early CSDH resolution. Adjunctive TXA may be an effective treatment option to reduce recurrence by enhancing CSDH resolution in the selective patients.
Key Words: Hematoma, subdural, chronic · Tranexamic acid · Recurrence.
INTRODUCTION
Chronic subdural hematoma (CSDH) is a common neurosurgical disease that affects the elderly population. The incidence of CSDH, estimated between 1.7 and 58 per 100000 persons per year, has been rising with the aging population because elderly patients often develop progressive cerebral atrophy, have comorbidities, and are treated with antiplatelet and anticoagulant drugs [ 2, 4- 6]. While surgery effectively eliminates or reduces hematoma, 5-30% of patients develop a recurrent hematoma [ 2, 7, 10, 12, 22]. Repeated surgery is still the main strategy for patients, although the risk factors for recurrence are still debated [ 2]. Adjunctive or primary use of multiple drugs, including steroids, statins, angiotensin-converting enzyme inhibitors, and antifibrinolytics, to mitigate the risk of recurrence has been examined in the past decades [ 4, 6, 7]. However, there is no consensus regarding the optimal postoperative medical management to reduce the recurrence of CSDH. Tranexamic acid (TXA) is an antifibrinolytic agent and a synthetic derivative of the lysine used to reduce bleeding in patients having trauma, surgery, and uncontrolled bleeding [ 7, 8]. The use of TXA to reduce CSDH effectively was reported in a small cohort study, which included three cases of adjunctive TXA use after surgery [ 9]. There were several other studies reporting the successful role of TXA as a primary treatment of CSDH or as a salvage medication at CSDH recurrence [ 11, 13, 15, 25]. Recent studies have reported that TXA administration delayed the CSDH recurrence and reduced hematoma volume faster [ 26, 27]. Although randomized trials are ongoing to reveal the efficacy of TXA, the role of TXA as an adjunctive medical treatment still lacks evidence [ 8, 29]. Therefore, this retrospective study aimed to reinforce the effectiveness of adjunctive TXA after hematoma evacuation in the view of early hematoma resorption. In addition, risk factors for the CSDH recurrence were investigated in a single-institute cohort.
MATERIALS AND METHODS
The study was approved by Institutional Review Board of Chungnam National University Hospital (No. CNUH-2020-07-066-002).
Patient population
Between January 2018 and September 2021, 254 patients with symptomatic CSDH were treated surgically at a single tertiary institute, in whom CSDH was diagnosed using initial brain computed tomography (CT) or magnetic resonance imaging scans. Patients whose postoperative hematoma changes were not traced because of the follow-up loss or emergent surgical or systemic complications were excluded. The data extracted from the medical records comprised demographic features, clinical presentation, preoperative Markwalder’s grading score (MGS) and Glasgow coma scale (GCS), history of the cardio-cerebrovascular disease (CCVD) with antithrombotics (AT) medications (e.g., anti-platelets and anti-coagulants), surgical and systemic complications, postoperative administration of TXA and/or statins, and adverse events during adjunctive medical treatment.
Treatment and patient grouping
In all patients, the surgery was a single burr-hole trephination with the placement of a subdural catheter for hematoma drainage. No craniotomy or twist drill surgery was performed. Although detailed surgical techniques varied among neurosurgeons, five neurosurgeons performed surgery following our institutional guideline : to place a subdural catheter immediately after the puncture of the pseudo-membrane of the CSDH through a single burr-hole site without routine saline irrigation. Typical CSDH patients underwent three postoperative CT scans : immediately after surgery, 24 to 48 hours after surgery before removing the subdural drainage catheter, and 1 week after surgery to confirm hospital discharge. None of the patients was prescribed steroids during the treatment period. Follow-up CT scans were obtained 2-3 weeks after the discharge and 1-month interval thereafter.
No additional adjunctive medical therapy was performed during the early study period. However, during the late study period, administration of oral TXA was considered only in the patients without a history of CCVD to avoid unexpected thrombotic adverse events. Since using oral TXA adjuvant for the first time in March 2020, 88 patients were enrolled, of which 33 patients were not subjected to the oral TXA due to a history of CCVD. Of the remaining patients, 75% (41 of 55 patients) were treated with oral TXA as an adjunctive medication. Patients who refused adjunctive medications were not treated with TXA. Therefore, among patients with no history of CCVD during the late period of the study, majority of patients received oral TXA 750 mg daily until the primary or secondary endpoint (TXA group), and the remaining patients were observed without TXA medication (non-TXA group). Whereas patients with a CCVD history and AT medication were grouped into the AT group and analyzed for comparison with the other two groups. All AT medications were discontinued after surgery and were not resumed until the hematoma was resolved. Patient selection and grouping are summarized in Fig. 1.
Primary and secondary outcomes
The primary endpoint of this study was the recurrence of CSDH, defined as the re-appearance or re-accumulation of CSDH requiring neurosurgical interventions, including repeat surgery, middle meningeal artery embolization, and intensive medical treatments. The decision regarding the clinical situation was made by experienced neurosurgeons.
The secondary endpoint was the resolution of CSDH. It was defined as complete or near-complete resorption of the CSDH with the recovery of neurological deficits. Radiologically, complete resorption was considered when the thickness of the CSDH in the follow-up CT scan was less than 5 mm. The near-complete resorption of CSDH was determined when CSDH was stationary in subsequent examinations, even if the hematoma thickness was close to 5 mm or more. In addition, the time to resolution, defined as the period between surgery and resolution of CSDH, was used in the Kaplan-Meier method. The serial thickness of the CSDH was also investigated to demonstrate changes in the CSDH over time.
Imaging evaluation
The data from initial and follow-up images were extracted including hematoma location (unilateral or bilateral), classification of CSDH (homogeneous, laminated, separated and trabecular), midline shift, and maximal thickness of CSDH [ 16]. To minimize measurement error, two neurosurgeons examined the thickness of CSDH according to the following agreement : 1) the maximal thickness was measured on a perpendicular line to the brain cortex (frontal or parietal lobe) from the inner table of the skull in axial, coronal and sagittal images; 2) sequential measurements of thickness were performed on the same axis of initial measurement as possible; and 3) the site of pneumocephalus was avoided to be measured. The paired values of the CSDH thickness, assessed independently by two examiners, were statistically evaluated.
Statistical analyses
Statistical analyses were performed using R software (version 4.1.2; R Project for Statistical Computing, Vienna, Austria) and SPSS software (version 18.0.0; SPSS Inc., Armonk, NY, USA) with a 2-sided p-value of 0.05 as the significance threshold in all tests. Categorical variables were presented as the number of patients with percentages. Normally distributed continuous variables were reported as mean with standard deviations, and non-parametric variables as median with interquartile ranges (IQRs). Three-group comparisons were conducted using Fisher’s exact test, one-way ANOVA, and the Kruskal-Wallis test. Tukey’s test was adopted as a post-hoc test comparing all possible pairs of mean values of groups to determine which specific pairs of groups were significantly different. The logistic regression analysis was used for determining risk factors for the primary outcome. The Kaplan-Meier method was used for survival analyses, and the log-rank test to compare treatment was conducted. For the primary and secondary endpoints, data censoring occurred when patients died of unrelated problems of study, and patients remained free of the entity being studied at the end of the follow-up period. The Cox proportional hazards model was used to perform the univariate and multivariate analyses. The measurement error of CSDH thickness was evaluated using the intraclass correlation coefficient (ICC). The CSDH thickness values were presented with line graphs as median values with IQR at each time points series (initial, 1 week, and 1, 2, 3, 4, and 6 months).
RESULTS
Of the 254 patients, 14 patients were excluded who underwent rescue craniotomy because of surgical complications (ipsilateral or contralateral acute subdural hematoma, n=3), died of systemic complications (pulmonary thromboembolism, n=2), exacerbated underlying extracranial malignancy (advanced gastric cancer, n=1), and were lost to follow-up due to unknown reasons (n=8). Therefore, a total of 240 patients was included in the analysis, consisting of 185 male (77.1%) and 55 female (22.9%), with a median age of 74 years (IQR, 64-81). During the median imaging follow-up period of 75 days (IQR, 44-126), 222 patients reached the primary or secondary endpoint, while 18 patients were lost to follow-up before the study endpoints. The median clinical follow-up time was 10 months (IQR, 4.7-23.6). At presentation, 98 patients had MGS grade 1, 132 had grade 2, and 10 had grade 3. The preoperative GCS scores varied from 8 to 15, and most patients (91.3%) scored 13 or more. Bilateral CSDH was identified in 78 patients (32.5%), and 48 patients (20.0%) underwent bilateral surgery at the initial presentation regarding hematoma volume and clinical status. Surgery was performed under general anesthesia in 209 patients (87.1%). The median maximal thickness before surgery was 21.6 mm (IQR, 18.1-24.7 mm) and the median thickness at hospital discharge (typically, 1 week after surgery) was 11.3 mm (IQR, 9.1-14.4 mm). The homogeneous type of CSDH was identified in 143 patients (59.6%), followed by trabecular (n=45, 18.8%), laminar (n=33, 13.8%), and separated (n=19, 7.9%) types.
A total of 85 patients (AT group, 35.1%) had AT because of a history of CCVD, which consisted of 64 patients having antiplatelets (including 17 patients with dual-platelets) and 21 patients having anti-coagulants (13 with warfarin and eight with direct-acting oral anticoagulants). Among 155 patients without a history of CCVD, TXA was administered as adjunctive therapy after surgery in 41 patients (TXA group, 16.9%). The remaining 114 patients were included in the non-TXA group (47.9%). In the TXA group, patients took TXA for median time of 49 days (range, 14-141). Neither new CCVD events after discontinuing AT medication nor TXA-related events occurred during the study period. After the surgery, statin administration was initiated or continued in 79 patients (32.9%).
The TXA, non-TXA, and AT groups were balanced in patient characteristics except for age, bilateral surgery, and statin administration ( Table 1). The median age was oldest in the AT group, followed by the TXA and non-TXA groups. In addition, bilateral surgery and postoperative statin prescriptions were more frequent in the TXA group.
Primary outcome
CSDH recurred in 16 patients (6.7%). Among these, 12 patients underwent repeat surgery, three underwent middle meningeal artery embolization, and one was managed conservatively. Multiple interventions were required in three patients. The recurrence rates were 2.4% in the TXA group (n=1), 7.0% in the non-TXA group (n=8), and 8.2% in the AT group (n=7). The recurrence rate was the lowest in the TXA group, but the difference was not statistically significant ( Fig. 2A). Recurrence-free survival was similar among the three groups ( Fig. 2B). The univariate and multivariate analyses revealed that patient demographics including age, sex, medical history, preoperative MGS, bilateral hematoma and surgery, preoperative hematoma thickness, and the use of TXA and/or statins were not related to recurrence ( Table 2). However, the homogeneous type of CSDH was a negative risk factor for recurrence in the multivariate analysis ( p=0.015; hazard ratio [HR], 0.259; 95% confidence interval [CI], 0.087-0.766). In patients with recurrence, the trabecular type (n=7) was the most prevalent hematoma type, followed by the homogeneous (n=5), laminar (n=2), and separated (n=2) types.
Secondary outcomes
Resolution of CSDH was achieved in 206 patients (85.8%), and the crude rates were not statistically different among TXA (39 of 41 patients, 95.1%), non-TXA (97 of 114 patients, 85.1%), and AT (70 of 85 patients, 82.4%) groups ( Fig. 2A). However, the median estimated time to resolution was significantly faster in the TXA group (51 days; 95% CI, 45.0-57.0) than the non-TXA (109 days; 95% CI, 102.3-115.7) and AT (88 days; 95% CI, 66.7-109.4) groups (log-rank test, p<0.001; Fig. 3A). In the multivariate analysis, TXA administration was a significant positive factor for achieving CSDH resolution ( p<0.001; HR, 3.323; 95% CI, 2.231-4.948; Table 3). In addition, less residual hematoma at discharge (i.e., maximal thickness of CSDH at one week after surgery) was also a significant prognostic factor for CSDH resolution ( p=0.004; HR, 0.943; 95% CI, 0.906-0.981). The maximal thickness values of CSDH over the follow-up period were measured in 1004 CT scans, and the ICC was 0.843 ( p=0.03; 95% CI, 0.452-0.933). CT scans at initial presentation and 1 week (a total of 480 CT scans) after surgery were available for all patients, and the number of reviewed CT scans decreased over time because patients reached primary or secondary endpoints or were lost to follow-up. In the TXA group, only two CT scans were available at 4 months and none at 6 months after surgery. The thickness was comparable among the three groups at the initial time and 1 week after surgery. However, 1 month after surgery, CSDH thickness in the TXA group decreased abruptly and showed a significant difference from that in the other groups ( p<0.001, Fig. 3B). The difference in the thickness between the TXA group and other groups was significant until the 4 months when almost all patients in the TXA group achieved CSDH resolution. Meanwhile, the non-TXA and AT groups showed almost same patterns of CSDH resolution.
DISCUSSION
In this study, TXA administration after CSDH surgery significantly facilitated the resorption of residual CSDH and resulted in the early CSDH resolution. Furthermore, CSDH recurrence was confirmed in only one case, although a statistical difference was not achieved. In this regard, the adjunctive use of TXA is expected to reduce recurrence by enhancing CSDH resolution. In addition, for the most part the results from the present study provide strengthened evidence for the CSDH treatment with TXA and confirm previous findings reported in the literature.
Understanding of the CSDH pathogenesis is important for the utilization of medical agents to manage CSDH. Although no definitive mechanism has been demonstrated for CSDH, several complex and multifocal mechanisms have been investigated. The pathway typically starts with an acute hematoma caused by minor trauma and venous injuries that trigger an inflammatory process in the subdural space. A membrane with fragile neocapillaries enveloping the blood clot forms over time. The blood clot may expand because of factors, such as hyper-fibrinolysis, accumulation of fibrin degradation products, osmotic gradient causing ingress of fluid into the hematoma, high concentration of vascular endothelial growth factor, and microbleeding of fragile capillaries [ 4, 24, 26]. CSDH is considered an angiogenic disease in which inflammation plays an important role. Plasmin, activated by a tissue plasminogen activator, is one of the key factors in the pathogenesis of CSDH. Plasmin activates the fibrinolytic system, which promotes the liquefaction and subsequent expansion of CSDH. In addition, the kallikrein system, which enhances inflammation and vascular permeability, is activated by plasmin [ 6, 24, 26]. TXA is a synthetic analog of lysine, which has an antifibrinolytic effect via inhibition of plasminogen activator and plasmin [ 3]. The advantages of TXA administration, such as reduced blood loss and the need for transfusion in neurosurgery, have been reported in the literature [ 1, 3, 17]. TXA may reduce increased vascular permeability of CSDH by inhibiting the hyper-fibrinolytic activity, resulting in a gradual resolution of the hematoma. Several authors have reported the positive effects of TXA as a primary or adjunctive treatment for CSDH, and several trials are ongoing [ 5, 8, 9, 25- 27, 31]. Multiple factors can contribute to recurrence after surgery, including coagulopathy, type and volume of the CSDH, surgical modalities, residual hematoma after surgery, pneumocephalus, and drain use [ 2, 16, 26, 27, 30]. In this study, as in the literature, the homogeneous appearance of CSDH was the only protective factor for recurrence [ 4, 30]. Recent multicenter study revealed that the separated type was a risk factor for CSDH recurrence [ 19]. However, in this cohort, a relationship between the recurrence and each CSDH type except for the homogeneous type was not statistically identified (data not shown). The overall low recurrence rate is thought to contribute to the inconsistency of these findings regarding CSDH types. One of the interesting findings of this study is that both the non-TXA and AT groups showed similar results in terms of the recurrence rate and CSDH resolution rate and speed. AT medication may not be associated with recurrence and resolution of CSDH. This finding is in accordance with other studies investigating the risk factors for CSDH recurrence [ 2]. Some authors have suggested that resumption of anticoagulant therapy is safe 72 hours after surgery, while antiplatelets should be withheld for at least 7 days following CSDH drainage [ 14, 22]. In this study, most patients previously taking AT medications did not resume medication until CSDH resolved, but some resumed medication early when deemed necessary. The present study results support the idea that preoperative AT therapy does not increase the risk of CSDH recurrence after evacuation; however, treatment should be tailored to each patient, considering specific cardiovascular risks. Recent trials investigating the role of adjunctive use of TXA to prevent CSDH recurrence after surgery failed to achieve statistical significant difference in the recurrence rates compared to the control groups [ 27, 31]. However, most studies consistently reported that patients with adjunctive TXA experienced lower recurrence rates (0% to 4.8%) [ 2, 9, 20, 26, 27, 31]. The reasons that every study including the present study did not reach a statistically significant difference in terms of CSDH recurrence may be the small number of patients in the treating group and the genuine low recurrence rate of CSDH surgery. The recurrence rates in the non-TXA and AT groups were 7.0% and 8.2%, respectively, which are lower than those reported in the literature. The difference in recurrence rates of the non-TXA and AT groups were 4.6% and 5.8%, respectively, compared to the TXA group (2.4% recurrence rate), and theoretically, 230 to 328 patients per group were required to detect a significant difference with >80% statistical power. A large cohort study or meta-analysis should be warranted to reveal significant differences [ 8, 28]. Despite this statistical aspect, adjunctive TXA use was expected to be effective in decreasing the recurrence of CSDH because it certainly reduced residual hematoma in time. Several studies universally reported a positive role of TXA in reducing residual CSDH during follow-up period [ 9, 26, 27, 31]. In this study, the resolution of CSDH was intentionally defined to verify the effect of reducing the time until complete hematoma resolution. Risk factor analyses for the resolution of CSDH revealed that the adjunctive TXA was the only significant factor in achieving early resolution. Furthermore, the effect of the adjunctive TXA to promote CSDH resolution was efficiently visualized as the line graphs in terms of residual hematoma volume. As a result, a significant time-saving effect of adjunctive TXA by accelerated resorption of the residual hematoma was successfully demonstrated.
Limitation
Our study was retrospective and limited by a small sample size and biases of a single-institution study. There was bias in patient selection, resulting in an imbalance in potential confounding variables including age, previous medication, and bilateral surgery, although these factors were not related to the primary and secondary outcomes in the multivariate analyses. In addition, possible confounding factors such as surgical methods, irrigation during surgery, open or close drainage, drug interaction and other demographic and radiological features that may affect outcomes should be considered. To validate and generalize the study results, further studies should be performed including multicenter studies and randomized trials as well as physiological experiments. Furthermore, the TXA group comprised patients without CCVD history. These patients were selected because of concerns about the possibility of TXA-associated thromboembolism, although several studies have reported the safety of TXA [ 3, 17, 18]. A subsequent trial is planned at our institution to validate the effectiveness and safety of adjunctive TXA regardless of CCVD history. Another weak point of this study was that the TXA administration was decided by the neurosurgeon’s preference rather than by definite selection criteria. In addition, statins, another potentially beneficial drug in reducing CSDH recurrence, was administered more frequently to the patients in the TXA group [ 4, 7, 21, 23, 24, 28]. TXA administration was conducted relatively recently in this cohort, and a more detailed investigation of drug history for the TXA group may be the reason for the imbalance. Although multivariate analysis revealed no relationship between statin administration and study outcomes, combined effects of TXA and statins need to be investigated. Therefore, caution should be exercised when interpreting the results of this study.
CONCLUSION
This retrospective study of patients with CSDH showed that the adjunctive use of TXA after burr-hole drainage is effective in achieving resolution of CSDH through faster hematoma reduction. TXA may have a favorable effect in reducing recurrence and can be administered safely in selected patients.
Acknowledgements
This work was supported by Chungnam National University.
Fig. 1.
Patient selection flow chart. CSDH : chronic subdural hematoma, CCVD : cardio-cerebrovascular disease, TXA : tranexamic acid, AT : antithrombotics. 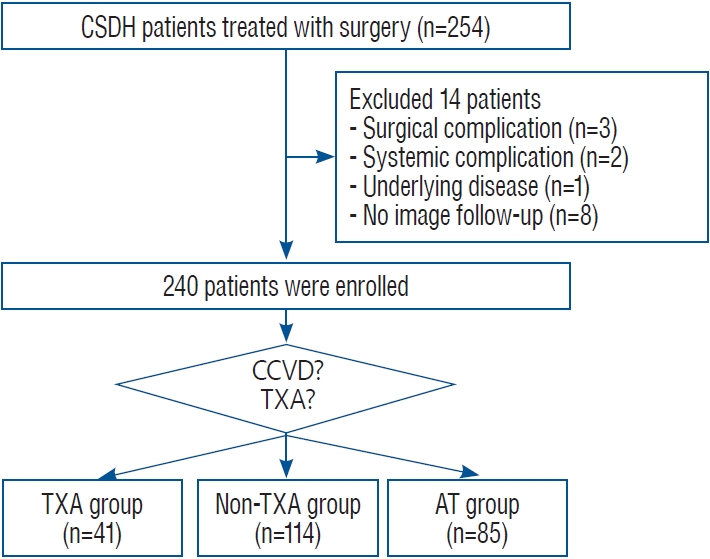
Fig. 2.
Summary of primary and secondary outcomes (A) and Kaplan-Meier plots of recurrence-free survival (B). F/U : follow-up, TXA : tranexamic acid, AT : antithrombotics. 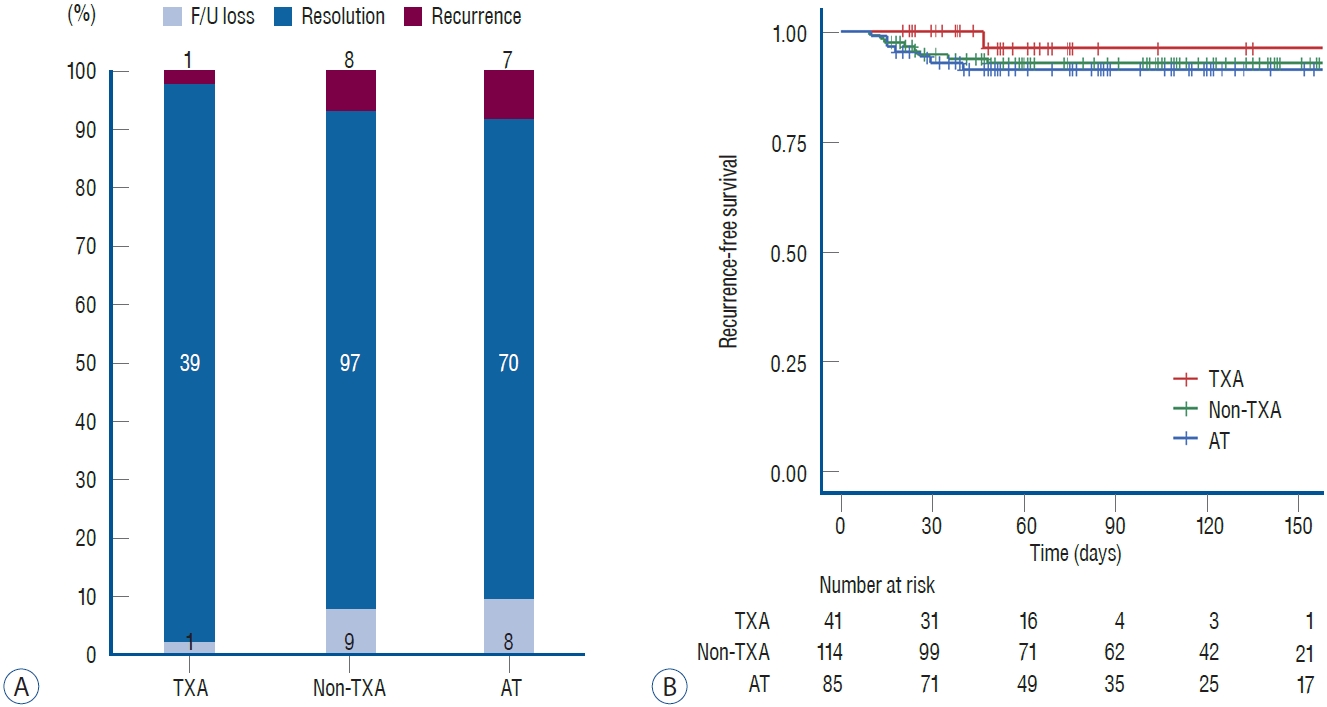
Fig. 3.
Kaplan-Meier plots of CSDH resolution (A) and line graphs of changes of CSDH thickness in time (B). *Statistically significant time points. CSDH : chronic subdural hematoma, TXA : tranexamic acid, AT : antithrombotics, N/A : not applicable. 
Table 1.
Patient characteristics and group comparison
|
TXA group (n=41) |
Non-TXA group (n=114) |
AT group (n=85) |
p-value |
|
Age (years) |
72 (65-83) |
71.5 (60-79) |
77 (68-82) |
0.019 |
|
Female |
8 (19.5) |
27 (23.7) |
20 (23.5) |
0.877 |
|
MGS grade 1 or 2 |
39 (95.1) |
112 (98.2) |
79 (92.9) |
0.137 |
|
GCS score ≥13 |
35 (85.4) |
109 (95.6) |
75 (88.2) |
0.052 |
|
Bilateral CSDH |
18 (43.9) |
36 (31.6) |
24 (28.2) |
0.199 |
|
Bilateral surgery |
14 (34.1) |
21 (18.4) |
13 (15.3) |
0.050 |
|
Statin administration |
27 (65.9) |
17 (14.9) |
35 (41.2) |
<0.001 |
|
Homogeneous type |
14 (34.1) |
44 (38.6) |
39 (45.9) |
0.380 |
|
Midline shift (mm) |
8.1 (4.8-11.2) |
8.9 (4.9-12.1) |
9.2 (5.8-13.2) |
0.349 |
|
Maximal thickness (mm) |
|
|
|
|
|
Initial |
20.9 (18.2-24.5) |
22.4 (18.1-25.3) |
20.6 (17.2-24.4) |
0.348 |
|
One week after surgery |
11.4 (8.3-13.8) |
11.4 (8.7-15.2) |
11.0 (9.5-13.6) |
0.805 |
Table 2.
Univariate and multivariate risk factor analyses for the recurrence
|
Variable |
Univariate analysis
|
Multivariate analysis
|
|
OR |
95% CI |
p-value |
OR |
95% CI |
p-value |
|
Age |
0.968 |
0.928-1.011 |
0.140 |
0.963 |
0.917-1.011 |
0.127 |
|
Male |
2.170 |
0.478-9.854 |
0.316 |
3.064 |
0.583-16.094 |
0.186 |
|
Antithrombotics |
0.687 |
0.246-1.915 |
0.473 |
0.751 |
0.219-2.574 |
0.649 |
|
MGS grade 1 or 2 |
0.628 |
0.075-5.290 |
0.669 |
0.427 |
0.041-4.444 |
0.476 |
|
Bilateral surgery |
0.733 |
0.226-2.383 |
0.606 |
2.755 |
0.525-14.451 |
0.231 |
|
Maximal thickness |
0.947 |
0.823-1.089 |
0.445 |
0.909 |
0.781-1.059 |
0.221 |
|
TXA administration |
3.261 |
0.419-25.404 |
0.259 |
2.012 |
0.173-23.409 |
0.577 |
|
Statin administration |
1.905 |
0.595-6.096 |
0.277 |
1.633 |
0.411-6.480 |
0.486 |
|
Homogeneous type |
0.283 |
0.095-0.843 |
0.023 |
0.189 |
0.055-0.645 |
0.008 |
Table 3.
Univariate and multivariate risk factor analyses for the resolution of hematoma
|
Variable |
Univariate analysis
|
Multivariate analysis
|
|
HR |
95% CI |
p-value |
HR |
95% CI |
p-value |
|
Age |
0.995 |
0.983-1.008 |
0.474 |
0.990 |
0.977-1.004 |
0.166 |
|
Male |
1.149 |
0.828-1.594 |
0.406 |
1.179 |
0.837-1.660 |
0.346 |
|
Antithrombotics |
0.843 |
0.631-1.128 |
0.250 |
1.146 |
0.809-1.624 |
0.443 |
|
MGS grade 1 or 2 |
0.595 |
0.244-1.451 |
0.254 |
0.641 |
0.257-1.603 |
0.342 |
|
Bilateral surgery |
1.071 |
0.749-1.530 |
0.708 |
1.043 |
0.719-1.512 |
0.824 |
|
Maximal thickness |
0.951 |
0.916-0.988 |
0.009 |
0.943 |
0.906-0.981 |
0.004 |
|
TXA administration |
2.865 |
1.987-4.129 |
<0.001 |
3.323 |
2.231-4.948 |
<0.001 |
|
Statin administration |
1.300 |
0.981-1.722 |
0.068 |
1.009 |
0.729-1.396 |
0.956 |
|
Homogeneous type |
0.852 |
0.641-1.133 |
0.271 |
0.946 |
0.696-1.287 |
0.725 |
References
1. Cheriyan T, Maier SP 2nd, Bianco K, Slobodyanyuk K, Rattenni RN, Lafage V, et al : Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J 15 : 752-761, 2015   2. Cofano F, Pesce A, Vercelli G, Mammi M, Massara A, Minardi M, et al : Risk of recurrence of chronic subdural hematomas after surgery: a multicenter observational cohort study. Front Neurol 11 : 560269, 2020    3. de Faria JL, da Silva Brito J, Costa E Silva LT, Kilesse CTSM, de Souza NB, Pereira CU, et al : Tranexamic acid in neurosurgery: a controversy indication-review. Neurosurg Rev 44 : 1287-1298, 2021    5. Edlmann E, Holl DC, Lingsma HF, Bartek J Jr, Bartley A, Duerinck J, et al : Systematic review of current randomised control trials in chronic subdural haematoma and proposal for an international collaborative approach. Acta Neurochir (Wien) 162 : 763-776, 2020    6. Holl DC, Volovici V, Dirven CMF, Peul WC, van Kooten F, Jellema K, et al : Pathophysiology and nonsurgical treatment of chronic subdural hematoma: from past to present to future. World Neurosurg 116 : 402-411.e2, 2018   7. Huang J, Gao C, Dong J, Zhang J, Jiang R : Drug treatment of chronic subdural hematoma. Expert Opin Pharmacother 21 : 435-444, 2020   8. Iorio-Morin C, Blanchard J, Richer M, Mathieu D : Tranexamic acid in chronic subdural hematomas (TRACS): study protocol for a randomized controlled trial. Trials 17 : 235, 2016    9. Kageyama H, Toyooka T, Tsuzuki N, Oka K : Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J Neurosurg 119 : 332-337, 2013   10. Kolias AG, Chari A, Santarius T, Hutchinson PJ : Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol 10 : 570-578, 2014    11. Kutty RK, Peethambaran AK, Anilkumar M : Conservative treatment of chronic subdural hematoma in HIV-associated thrombocytopenia with tranexamic acid. J Int Assoc Provid AIDS Care 16 : 211-214, 2017    13. Lodewijkx R, Immenga S, van den Berg R, Post R, Westerink LG, Nabuurs RJA, et al : Tranexamic acid for chronic subdural hematoma. Br J Neurosurg 35 : 564-569, 2021   14. Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ : Evidence based diagnosis and management of chronic subdural hematoma: a review of the literature. J Clin Neurosci 50 : 7-15, 2018   15. Mikkelsen R, Anker-Møller T, Hvas AM, Sunde N : A case of tranexamic acid as adjunctive treatment for chronic subdural hematoma with multiple recurrences. Am J Case Rep 18 : 995-999, 2017    16. Nakaguchi H, Tanishima T, Yoshimasu N : Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg 95 : 256-262, 2001   17. Ng W, Jerath A, Wąsowicz M : Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 47 : 339-350, 2015   18. Ngaage DL, Bland JM : Lessons from aprotinin: is the routine use and inconsistent dosing of tranexamic acid prudent? Meta-analysis of randomised and large matched observational studies. Eur J Cardiothorac Surg 37 : 1375-1383, 2010   19. Oh HJ, Seo Y, Choo YH, Kim YI, Kim KH, Kwon SM, et al : Clinical characteristics and current managements for patients with chronic subdural hematoma : a retrospective multicenter pilot study in the Republic of Korea. J Korean Neurosurg Soc 65 : 255-268, 2022    20. Oka K, Toyooka T, Kageyama H, Tsuzuki N : Effectiveness of antifibrinolytic therapy after surgery for chronic subdural hematoma. J Neurosurg 119 : A550, 2013
21. Qiu S, Zhuo W, Sun C, Su Z, Yan A, Shen L : Effects of atorvastatin on chronic subdural hematoma: a systematic review. Medicine (Baltimore) 96 : e7290, 2017   22. Ryu SM, Yeon JY, Kong DS, Hong SC : Risk of recurrent chronic subdural hematoma associated with early warfarin resumption: a matched cohort study. World Neurosurg 120 : e855-e862, 2018   23. Scerrati A, Visani J, Ricciardi L, Dones F, Rustemi O, Cavallo MA, et al : To drill or not to drill, that is the question: nonsurgical treatment of chronic subdural hematoma in the elderly. A systematic review. Neurosurg Focus 49 : E7, 2020  24. Soleman J, Nocera F, Mariani L : The conservative and pharmacological management of chronic subdural haematoma. Swiss Med Wkly 147 : w14398, 2017    25. Stary JM, Hutchins L, Vega RA : Tranexamic acid for recurring subdural hematomas following surgical evacuation: a clinical case series. J Neurol Surg A Cent Eur Neurosurg 77 : 422-426, 2016   26. Tanweer O, Frisoli FA, Bravate C, Harrison G, Pacione D, Kondziolka D, et al : Tranexamic acid for treatment of residual subdural hematoma after bedside twist-drill evacuation. World Neurosurg 91 : 29-33, 2016   27. Wan KR, Qiu L, Saffari SE, Khong WXL, Ong JCL, See AA, et al : An open label randomized trial to assess the efficacy of tranexamic acid in reducing post-operative recurrence of chronic subdural haemorrhage. J Clin Neurosci 82 : 147-154, 2020   28. Wang X, Song J, He Q, You C : Pharmacological treatment in the management of chronic subdural hematoma. Front Aging Neurosci 13 : 684501, 2021    29. Workewych A, Callum J, Saarela O, Montanera W, Cusimano MD : Tranexamic acid in the treatment of residual chronic subdural hematoma: a single-centre, randomized controlled trial (TRACE). J Neurotrauma 35 : A244-A245, 2018
30. Xu FF, Chen JH, Leung GK, Hao SY, Xu L, Hou ZG, et al : Quantitative computer tomography analysis of post-operative subdural fluid volume predicts recurrence of chronic subdural haematoma. Brain Inj 28 : 1121-1126, 2014   31. Yamada T, Natori Y : Prospective study on the efficacy of orally administered tranexamic acid and goreisan for the prevention of recurrence after chronic subdural hematoma burr hole surgery. World Neurosurg 134 : e549-e553, 2020  
|
|



















