Kim, Shim, Choo, Kim, Kim, and Ha: Protein Requirement Changes According to the Treatment Application in Neurocritical Patients
Abstract
Objective
Exploring protein requirements for critically ill patients has become prominent. On the other hand, considering the significant impact of coma therapy and targeted temperature management (TTM) on the brain as well as systemic metabolisms, protein requirements may plausibly be changed by treatment application. However, there is currently no research on protein requirements following the application of these treatments. Therefore, the aim of this study is to elucidate changes in patients’ protein requirements during the application of TTM and coma therapy.
Methods
This study is a retrospective analysis of prospectively collected data from March 2019 to May 2022. Among the patients admitted to the intensive care unit, those receiving coma therapy and TTM were included. The patient’s treatment period was divided into two phases (phase 1, application and maintenance of coma therapy and TTM; phase 2, tapering and cessation of treatment). In assessing protein requirements, the urine urea nitrogen (UUN) method was employed to estimate the nitrogen balance, offering insight into protein utilization within the body. The patient’s protein requirement for each phase was defined as the amount of protein required to achieve a nitrogen balance within ±5, based on the 24-hour collection of UUN. Changes in protein requirements between phases were analyzed.
Results
Out of 195 patients, 107 patients with a total of 214 UUN values were included. The mean protein requirement for the entire treatment period was 1.84±0.62 g/kg/day, which is higher than the generally recommended protein supply of 1.2 g/kg/day. As the treatment was tapered, there was a statistically significant increase in the protein requirement from 1.49±0.42 to 2.18±0.60 in phase 2 (p<0.001).
Conclusion
Our study revealed a total average protein requirement of 1.84±0.62 g during the treatment period, which falls within the upper range of the preexisting guidelines. Nevertheless, a notable deviation emerged when analyzing the treatment application period separately. Hence, it is recommended to incorporate considerations for the type and timing of treatment, extending beyond the current guideline, which solely accounts for the severity by disease.
Key Words: Neurocritical care · Protein requirement · Nitrogen balance · Nutrition support · Urine urea nitrogen.
INTRODUCTION
Exploring protein requirements for critically ill patients has become prominent. Adequate protein intake can reduce mortality [ 20] and prevent various complications such as organ failure [ 23], sarcopenia [ 24], immune compromise [ 5], and failure of wound healing [ 26], leading to post-intensive care syndrome [ 14] and ultimately impacting functional outcomes. In this respect, current nutritional guidelines recommend adequate amounts of protein for critical patients, apart from appropriate calorie suggestions. The American Society for Parenteral and Enteral Nutrition (ASPEN) recommends a protein intake of between 1.2 and 2 g/kg of body weight per day, based on the severity of the disease [ 8]. However, the above guidelines have several limitations. Firstly, the suggested protein intake range (1.2-2.0 g/kg/day) is broad, which may lead to confusion in determining the exact protein requirements for critically ill patients. Secondly, apart from the severity of the disease itself, the patient’s individual severity changes with each treatment phase, making it questionable to set a fixed protein intake. The third limitation is that the existing guidelines do not consider the specific factors crucial for neurocritical patients.
Measuring protein requirements in patients with brain injuries poses several challenges. One is the impact of the brain itself, which accounts for 20% of total energy expenditure (TEE) and may be compromised [ 12]. Additionally, protein catabolism due to the systemic inflammatory response following brain damage must also be considered [ 7]. Yet, these tribulations do not stand alone. The implementation of coma therapy or targeted temperature management (TTM) introduces further complexities, as the therapy reduces the metabolic rate of the brain to prevent secondary neurological damage, thereby affecting the patient’s basal metabolic rate [ 1, 22]. Collectively, these factors impede the study of protein requirements in neurocritical patients. To date, studies on the protein requirements of neurocritical patients have primarily been limited to traumatic brain injury (TBI) [ 17], with a lack of research considering individual severity or the treatment chronomically. This study aims to identify changes in protein requirements during the process of neurocritical treatment, which includes coma therapy and TTM. We hypothesized that protein requirements would decrease during these treatments and increase during weaning.
MATERIALS AND METHODS
Patient consent was waived under Institutional Review Board of Seoul National University Hospital approval (2209-076-1358) for this retrospective study.
Study population
We retrospectively identified data collected prospectively from patients who were admitted to our intensive care unit (ICU) and underwent coma therapy, or TTM, for more than 72 hours from March 2019 to May 2022. Patients with oliguria or chronic kidney disease making urine collection challenging, as well as those lacking sufficient data for each treatment phase established in the study, were excluded.
Baseline characteristics and clinical information
Baseline characteristics, including age, sex, initial body weight and height, and medical history (e.g., diabetes mellitus, hypertension, dyslipidemia, heart disease, stroke, and cancer), were evaluated. In addition, we obtained clinical information regarding the initial Glasgow coma scale (GCS), length of stay in the ICU, starting time and ending time of TTM, and coma therapy.
Treatment strategies
Application of coma therapy and TTM
When patients with a GCS score of less than 8 were admitted to the ICU, intracranial pressure (ICP) monitoring was implemented if there was evidence of ICP elevation within brain computed tomography (CT). If the ICP remained above 22 mmHg despite appropriate baseline management, such as head elevation to 30-45 degrees, maintaining normotension, normothermia, normocapnia, euvolemia, and osmotic therapy, coma therapy using continuous infusions of thiopental or propofol was applied. Simultaneously, if no contraindications, such as coagulopathy, were present, TTM was utilized to lower the patient’s body temperature to 35 degrees Celsius. In cases where refractory ICP elevation persisted, further lowering of the body temperature, down to 34 or even 33 degrees Celsius, was considered. The study was conducted exclusively on patients who had successfully undergone coma therapy and TTM without experiencing severe complications.
Tapering of treatment
After patients receiving coma therapy and TTM had their ICP well controlled on the monitors for a certain period (24-48 hours), a brain CT was performed to confirm the absence of additional threats to ICP elevation, such as delayed cerebral ischemia or hemorrhage re-expansion. Subsequently, the tapering of treatment was initiated. Coma therapy involved gradually reducing the drug concentration by 1 hour, while TTM increased the temperature by 0.05 degrees Celsius per hour until reaching a target of 36 degrees Celsius. During the progressive tapering process, if there was no significant increase in ICP, coma therapy transitioned to light sedation using dexmedetomidine. For TTM, after reaching 36 degrees Celsius, normothermia was maintained for 24 hours, and if the ICP remained tolerable, the same method was used to raise the temperature to 37 degrees Celsius before discontinuation. The duration of the treatment application was approximately 7 to 10 days.
Protein requirement monitoring with urine urea nitrogen (UUN)
We analyzed protein requirements using UUN measurements from urine collected via a Foley catheter over a 24-hour period. During the treatment application and maintenance phases, one test was conducted, and an additional test was performed during the tapering phase after achieving the treatment goals. We divided the entire treatment period into two groups, namely phase 1 and phase 2, for comparison. Phase 1 was defined as the period from the initiation of coma therapy and TTM until just before tapering, while phase 2 was defined as the period from the start of tapering until the discontinuation of coma therapy and TTM. According to Bingham [ 4], the patient’s ‘protein requirement’ was defined as the protein mass needed to achieve a nitrogen balance within ±5 [ 4]. In this study, we adopted the same approach to calculate the patient’s protein requirement. The formula used for the calculation is as follows : nitrogen balance (g/day) = protein intake (g) / 6.25 - (UUN + 4).
Statistical analysis
We compared the protein requirement for each phase using the Wilcoxon rank-sum test and performed the Dun’s test for post hoc analyses. The same methods were used for subgroup analyses by diagnoses. All statistical analyses were performed using R software (The R Foundation, Vienna, Austria).
RESULTS
Patient characteristics
Data from 195 patients was collected between March 2019 and May 2022. Out of these, 88 patients who met the exclusion criteria were excluded, including three with renal impairment and 85 with insufficient UUN data for each phase. Ultimately, a total of 214 UUN data points from 107 patients were utilized for the analyses.
Among the patients, 44.9% were male (n=48), and the mean age was 56.6±17.8 years. Twenty-five patients (23.4%) had a history of diabetes mellitus, 34 (31.8%) had hypertension, 11 (10.3%) had heart disease, and 15 (14.0%) had a history of stroke. Twelve patients (11.2%) had a history of cancer.
Regarding the specific diagnoses, 28 patients (26.7%) were diagnosed with subarachnoid hemorrhage (SAH), 27 (25.7%) with intracerebral hemorrhage (ICH), 22 (21.0%) with brain tumor, 18 (17.1%) with TBI, five (4.8%) with ischemic stroke, and three (2.9%) with pure intraventricular hemorrhage. The median initial GCS score was 9 (range, 6-14), and the modified Rankin scale score was 4 (range, 3-5). The mean length of stay in the ICU was 19.5±13.9 days ( Table 1).
Estimated protein requirement
Out of the total 214 UUN data collected from 107 patients, the mean value of protein requirements, as measured by UUN throughout the entire period, was 1.84±0.62 g/kg/day. During phase 1, the protein requirement was 1.49±0.42 g/kg/day, while in phase 2, as the treatment was tapered, it increased to 2.18±0.60 g/kg/day. This increase demonstrates statistical significance ( p<0.0001, Fig. 1). In the subgroup analyses based on specific diagnoses, consistent findings were observed. For patients diagnosed with SAH, the protein requirement during phase 1 was 1.39±0.31 g/kg/day, which increased to 2.16±0.55 g/kg/day during phase 2. Similarly, in patients with ICH, the protein requirement was 1.56±0.49 g/kg/day in phase 1 and increased to 2.32±0.80 g/kg/day in phase 2. Patients with brain tumors demonstrated a protein requirement of 1.62±0.54 g/kg/day in phase 1, rising to 2.17±0.49 g/kg/day in phase 2. Likewise, those with TBI exhibited a protein requirement of 1.42±0.27 g/kg/day in phase 1 and 2.14±0.48 g/kg/day in phase 2. In all subgroups, a significant increase was observed between phases, demonstrating the clinical relevance of these findings ( Fig. 2).
DISCUSSION
Importance of protein in critical patients
Protein is the essential macronutrient for wound healing, immune function, and maintaining body mass in critically ill patients, and thus is more than just a source of calories [ 19]. Compher and colleagues [ 8] showed that the odds of death decreased by 6.6% with each 10% increase in protein intake. Furthermore, adequate protein intake was also found to help reduce the time to wean from the ventilator and shorten the length of stay in the ICU [ 16]. A lot of guidelines emphasize protein intake, recognizing its significance beyond providing calories. According to the 2016 ASPEN guidelines, critically ill patients require 1.2 to 2.0 g/kg of protein per day, with higher protein intake recommended for severe stress situations such as burns and multiple fractures [ 19]. Specifically, for TBI, the guideline suggests a protein intake as high as 1.5-2.5 g/kg/day, which aligns with our findings. The 2019 ESPEN guideline also recommends supplying at least 1.3 g/kg of protein daily to critically ill patients [ 25]. In general, critically ill patients are likely to fall into a catalytic state in the acute phase, so the demand for systemic and protein metabolism increases [ 11]. However, some reports showed that patients receive only 25-60% of their protein requirements on average during intensive care, which was also associated with a poor prognosis [ 28]. While neurocritical patients have more stress factors than other critical patients, it has been confirmed that they experience negative nitrogen balance and protein shortage problems [ 2, 3, 7, 15, 27].
Neurocritical treatments and metabolism
Since the brain is a high-nutrient-metabolic organ that uses 20% of TEE, changes in brain metabolism can also lead to changes in the patient’s TEE. This is related not only to protein catabolism caused by the systemic inflammatory response along with brain damage but also to the intensity level of treatment applied [ 3, 15, 27]. Neurocritical treatments, such as coma therapy, deep sedation, or TTM, lower the brain metabolism rate and decrease ICP. Previous studies have shown that the resting metabolic rate is reduced by 25% when propofol sedation is applied [ 9, 21]. Body temperature is also a factor that affects energy consumption. It is known that the cerebral metabolic rate decreases by 5% to 7% and energy consumption by 10% to 13% with every drop of a degree Celsius in the body temperature [ 10, 18]. Coma therapy is also known to reduce systemic metabolism and body temperature [ 13]. Nevertheless, limited research has been conducted regarding the impact of these therapies on protein metabolism. Through the ICP-guided treatment, we successfully maintained ICP and optimal cerebral perfusion pressure in most patients included in our study. Despite the adequate utilization of coma therapy and TTM, the protein requirements identified in our study (1.50±0.44 g/kg/day) fell within the upper range of the preexisting guideline, as suggested by ASPEN. These requirements would have been higher if coma therapy or TTM were not applied. Consequently, if nutrition supply is planned based on the overall reduced calorie requirements, it becomes difficult to supply sufficient protein. Buckley and Dickerson [ 6] also highlighted that patients may be at risk of protein deficiency during propofol sedation.
Significant increase in protein requirement during treatment weaning
Considering the impact of protein on neurological patients, it is essential to probe protein requirements with regard to brain metabolism. In our study, a significant increase in protein requirements was observed between phases 1 and 2. Therefore, it would be more advisable to monitor protein requirements at each treatment phase than at regular intervals. By measuring protein requirements and supplying an adequate amount, we can minimize protein shortages. Furthermore, while the overall caloric demand diminishes due to the effects of interventions such as coma therapy and TTM, the protein requirement during phase 1 remains notably high (1.49±0.42). Given this, it becomes necessary to contemplate how to incorporate these findings into the patient’s nutritional plan. Of course, research on functional outcomes should precede this.
Limitations
First, as a retrospective study, it is crucial to acknowledge that this research is inherently constrained by some missing data. Second, conducting a comprehensive analysis of factors that might influence UUN, such as blood transfusions, vasopressor usage, and patients’ digestive capacity, was challenging due to methodological complexities. Third, in the cases of relatively mild patients who did not undergo coma therapy and TTM in neurocritical care or non-neurocritical patients, we did not perform early and thorough UUN analysis, so there were no control groups to confirm the increased protein requirement and its changes. Fourth, we did not observe an association between protein supplementation using UUN and clinical outcomes. Nevertheless, this study lays the groundwork for future investigations that explore the potential benefits of tailoring protein supplementation based on individual patients’ specific protein requirements to enhance their clinical outcomes.
CONCLUSION
In neurocritical patients, especially those undergoing treatments like coma therapy or TTM that influence metabolism, significant changes in protein requirements were observed. Considering these findings, it may be necessary to reevaluate the current protein supply guidelines that solely rely on the ‘severity index of the disease’. Further assessment is warranted to determine the appropriateness and relevance of the existing protein guidelines considering these significant variations in protein needs.
Fig. 1.
Protein requirements according to neurocritical treatment phases. During the active implementation of targeted temperature management and coma therapy in phase 1, the protein requirement was 1.49±0.42 g/kg/day. However, in phase 2, as the intensive treatment was tapered, it increased to 2.18±0.60 g/kg/day (****p<0.0001). The unit of measurement is g/kg/day. 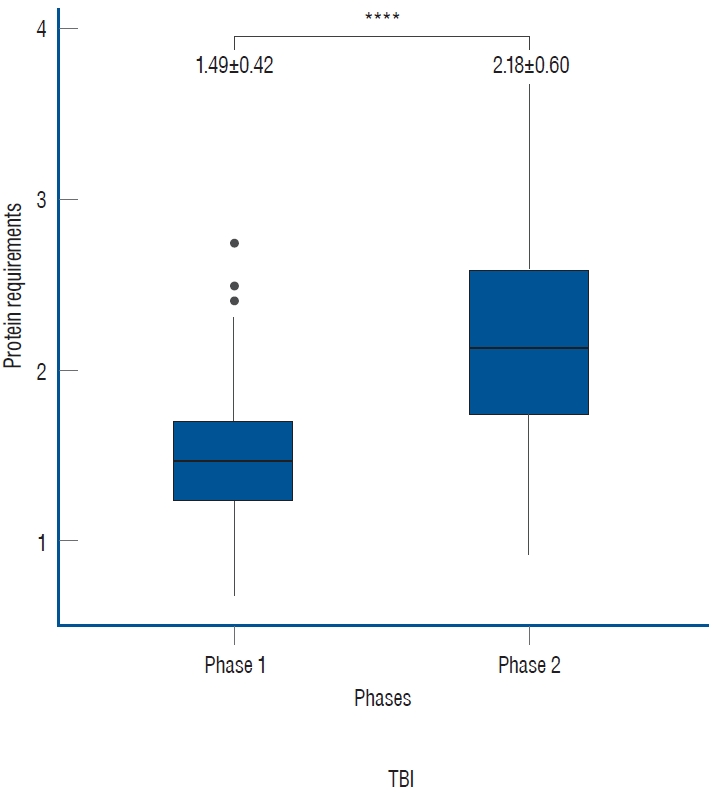
Fig. 2.
Subgroup analyses of protein requirements based on specific diagnoses. In all subgroup analyses, the trends in phases 1 and 2 were consistent with those observed in the overall patient population. The unit of measurement is g/kg/day (***p<0.001, ****p<0.0001). TBI : traumatic brain injury, SAH : subarachnoid hemorrhage, ICH : intracerebral hemorrhage. 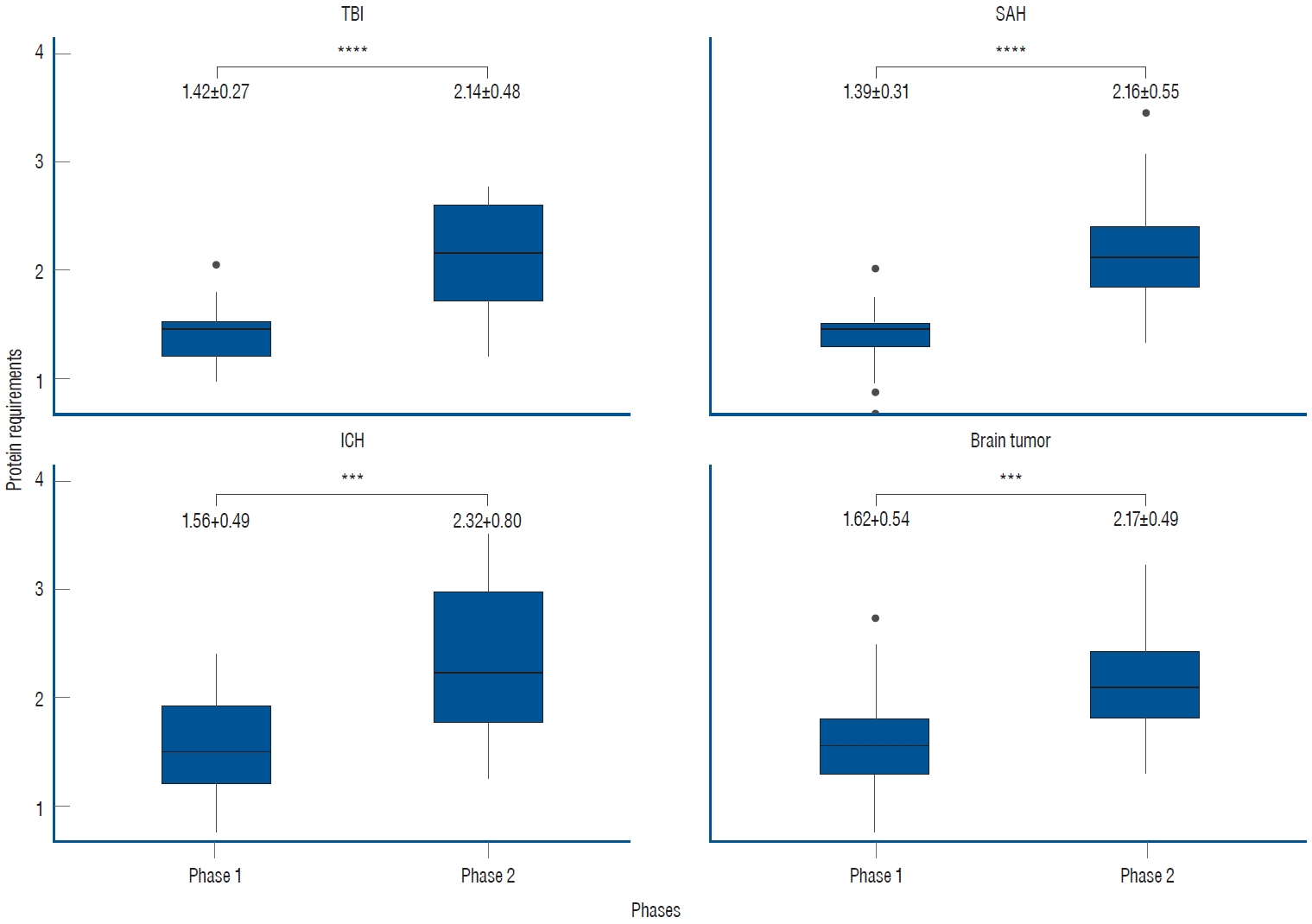
Table 1.
Patient characteristics (n=107)
|
Characteristic |
Value |
|
Sex |
|
|
Male |
48 (44.9) |
|
Female |
59 (55.1) |
|
Age (years) |
56.6 ±17.8 |
|
Initial body weight (kg) |
62.8±15.2 |
|
Initial height (cm) |
163.0±9.9 |
|
Initial BMI (kg/m2) |
23.4±4.2 |
|
Underlying disease |
|
|
Diabetes mellitus |
25 (23.4) |
|
Hypertension |
34 (31.8) |
|
Dyslipidemia |
23 (21.5) |
|
Heart disease |
11 (10.3) |
|
Previous stroke |
15 (14.0) |
|
Cancer |
12 (11.2) |
|
Diagnosis |
|
|
Subarachnoid hemorrhage |
28 (26.7) |
|
Intracerebral hemorrhage |
27 (25.7) |
|
Traumatic brain injury |
18 (17.1) |
|
Intraventricular hemorrhage, pure |
3 (2.9) |
|
Ischemic stroke |
5 (4.8) |
|
Other vascular diseases |
2 (1.9) |
|
Brain tumor |
22 (21.0) |
|
Initial GCS |
9 (6-14) |
|
Initial mRS |
4 (3-5) |
|
ICU LOS (days) |
19.5±13.9 |
References
1. Ashcraft CM, Frankenfield DC : Energy expenditure during barbiturate coma. Nutr Clin Pract 28 : 603-608, 2013    2. Badjatia N, Monahan A, Carpenter A, Zimmerman J, Schmidt JM, Claassen J, et al : Inflammation, negative nitrogen balance, and outcome after aneurysmal subarachnoid hemorrhage. Neurology 84 : 680-687, 2015    3. Bidkar PU : Nutrition in neuro-intensive care and outcomes. J Neuroanaesth Crit Care 3 : S70-S76, 2016  4. Bingham SA : Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr 133 Suppl 3 : 921S-924S, 2003  5. Bourke CD, Berkley JA, Prendergast AJ : Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol 37 : 386-398, 2016    8. Compher C, Bingham AL, McCall M, Patel J, Rice TW, Braunschweig C, et al : Guidelines for the provision of nutrition support therapy in the adult critically ill patient: the American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 46 : 12-41, 2022    9. Dickerson RN, Roth-Yousey L : Medication effects on metabolic rate: a systematic review (part 1). J Am Diet Assoc 105 : 835-843, 2005   10. Erecinska M, Thoresen M, Silver IA : Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab 23 : 513-530, 2003    12. Fisher N, Uhler ME : Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Philadelphia : Lippincott Williams & Wilkins, 1999
13. Fuhrman FA : The effect of body temperature on the duration of barbiturate anesthesia in mice. Science 105 : 387-388, 1947   14. Heyland DK, Mourtzakis M : Malnutrition in Critical Illness: Implications, Causes, and Therapeutic Approaches in Stevens RD, Hart N, Herridge MS (eds) : Textbook of Post-ICU Medicine: The Legacy of Critical Care. Oxford : Oxford University Press, 2014, pp.401-416
15. Heyland DK, Weijs PJ, Coss‐Bu JA, Taylor B, Kristof AS, O’Keefe GE, et al : Protein delivery in the intensive care unit: optimal or suboptimal? Nutr Clin Pract 32 : 58S-71S, 2017    16. Ingadottir AR, Beck AM, Baldwin C, Weekes CE, Geirsdottir OG, Ramel A, et al : Association of energy and protein intakes with length of stay, readmission and mortality in hospitalised patients with chronic obstructive pulmonary disease. Br J Nutr 119 : 543-551, 2018   18. McCall M, Jeejeebhoy K, Pencharz P, Moulton R : Effect of neuromuscular blockade on energy expenditure in patients with severe head injury. JPEN J Parenter Enteral Nutr 27 : 27-35, 2003   19. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al : Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 40 : 159-211, 2016  20. Mogensen KM, Robinson MK, Casey JD, Gunasekera NS, Moromizato T, Rawn JD, et al : Nutritional status and mortality in the critically ill. Crit Care Med 43 : 2605-2615, 2015   21. Moritz F, Petit J, Kaeffer N, Oksenhendler G, Papion H, Hecketsweiler B, et al : Metabolic effects of propofol and flunitrazepam given for sedation after aortic surgery. Br J Anaesth 70 : 451-453, 1993   22. Polderman KH : Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 37( 7 Suppl):S186-S202, 2009   23. Sato J, Inaba H, Hirasawa H, Mizuguchi T : Metabolic changes associated with malnutrition in the patients with multiple organ failure. J Anesth 7 : 276-286, 1993    24. Sieber CC : Malnutrition and sarcopenia. Aging Clin Exp Res 31 : 793-798, 2019    25. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al : ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 38 : 48-79, 2019   26. Stechmiller JK : Understanding the role of nutrition and wound healing. Nutr Clin Pract 25 : 61-68, 2010    27. Tripathy S : Nutrition in the neurocritical care unit. J Neuroanaesth Crit Care 2 : 88-96, 2015  28. Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux R N MC, Delarue J, et al : Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr 24 : 502-509, 2005  
|
|


















