Lee, Kim, Jung, Jung, Moon, Kim, Park, and Lim: Two-Day Fraction Gamma Knife Radiosurgery for Large Brain Metastasis
Abstract
Objective
We investigated how treating large brain metastasis (LBM) using 2-day fraction Gamma Knife radiosurgery (GKRS) affects tumor control and patient survival. A prescription dose of 10.3 Gy was applied for 2 consecutive days, with a biologically effective dose equivalent to a tumor single-fraction dose of 16.05 Gy and a brain single-fraction dose of 15.12 Gy.
Methods
Between November 2017 and December 2021, 42 patients (mean age, 68.3 years; range, 50-84 years; male, 29 [69.1%]; female, 13 [30.9%]) with 44 tumors underwent 2-day fraction GKRS to treat large volume brain metastasis. The main cancer types were non-small cell lung cancer (n=16), small cell lung cancer (n=7), colorectal cancer (n=7), breast cancer (n=3), gastric cancer (n=2), and other cancers (n=7). Twenty-one patients (50.0%) had a single LBM, 19 (46.3%) had a single LBM and other metastases, and two had two (4.7%) large brain metastases. At the time of the 2-day fraction GKRS, the tumors had a mean volume of 23.1 mL (range, 12.5-67.4). On each day, radiation was administered at a dose of 10.3 Gy, mainly using a 50% isodose-line.
Results
We obtained clinical and magnetic resonance imaging follow-up data for 34 patients (81%) with 35 tumors, who had undergone 2-day fraction GKRS. These patients did not experience acute or late radiation-induced complications during follow-up. The median and mean progression-free survival (PFS) periods were 188 and 194 days, respectively. The local control rates at 6, 9, and 12 months were 77%, 40%, and 34%, respectively. The prognostic factors related to PFS were prior radiotherapy (p=0.019) and lung cancer origin (p=0.041). Other factors such as tumor volumes, each isodose volumes, and peri-GKRS systemic treatment were not significantly related to PFS. The overall survival period of the 44 patients following repeat stereotactic radiosurgery (SRS) ranged from 15-878 days (median, 263±38 days; mean, 174±43 days) after the 2-day fraction GKRS. Eight patients (18.2%) were still alive.
Conclusion
Considering the unsatisfactory tumor control, a higher prescription dose should be needed in this procedure as a salvage management. Moreover, in the treatment for LBM with fractionated SRS, using different isodoses and prescription doses at the treatment planning for LBMs should be important. However, this report might be a basic reference with the same fraction number and prescription dose in the treatment for LBMs with frame-based SRS.
Key Words: Gamma knife radiosurgery · Large brain metastasis · Radiation dose fractionation · Stereotactic radiosurgery.
INTRODUCTION
Large brain metastasis (LBM) have a maximum diameter of ≥2-4 cm or a volume of ≥4-15 cm 3. Traditionally, these tumors are treated through surgery and whole brain radiation therapy (WBRT) [ 1, 2, 10, 17, 23, 25, 28]. Although surgery and consecutive postoperative radiation therapy are the main treatment options for patients with LBM, many of them do not qualify for surgery because of their general condition, Karnofsky performance scale, age and comorbidities, tumor location or uncontrolled primary cancers, and/or extracranial metastasis [ 25]. Therefore, other treatment options, such as stereotactic radiosurgery (SRS), are needed for LBM patients. Although the use of single-fraction SRS to treat small lesions can effectively control tumors and spare normal tissue, the capacity to safely target large tumors with adequate doses in a single fraction is limited [ 15, 16]. Recently, the use of fractionated SRS or a combination of these modalities with conventional treatments has been introduced for LBM management [ 1, 5, 6, 13, 20, 23, 37]. However, they vary widely in prescription dose and fraction number. In this study, we used 2-day fraction Gamma Knife radiosurgery (GKRS) to treat LBM at a radiation dose of 10.3 Gy per day.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board (IRB) of Chonnam National University Hwasun Hospital (IRB No. CNUHH-2023-206).
Between November 2017 and December 2021, 42 patients (mean age, 68.3 years; range, 50-84 years; male, 29 [69.1%]; female, 13 [30.9%]) with 44 tumors underwent 2-day fraction GKRS for large volume brain metastasis. The main cancer types were non-small cell lung cancer (n=16), small cell lung cancer (n=7), colorectal cancer (n=7), breast cancer (n=3), gastric cancer (n=2), and other cancers (ampulla of Vater cancer, endometrial cancer, hepatocellular carcinoma, pancreatic cancer, pharyngeal cancer, renal cell carcinoma, and small bowel cancer, n=7). Twenty-one patients (50.0%) had one large-volume metastasis, 19 (46.3%) had one LBM and other metastases, and two (4.7%) had large-volume metastases. Of the 44 tumors, most tumors (n=38) were newly developed, and of the remaining six, two locally recurred after GKRS, two recurred after WBRT, and two recurred after both managements ( Table 1). In this study, LBM was defined as metastasis with a diameter of >3 cm and that occurred only in the cerebral or cerebellar hemispheres.
Two-day fraction GKRS
Two-day fraction GKRS was administered to patients with large brain metastases. On the first day, a Leksell stereotactic frame type G was fixed on the patient’s head under local anesthesia, followed by the administration of a contrast medium and stereotactic acquisition of magnetic resonance T2-weighted, post-contrast images. The images were then transferred to a planning workstation for GKRS. For each patient, the 3-dimensional treatment plan was individualized using the Leksell GammaPlan version 5.31, 5.32, 5.34, or 9.0 (Elekta Instruments, Stockholm, Sweden). A Leksell gamma knife Perfexion (Elekta Instruments) was used for irradiation. After the treatment, the patients were moved back to the ward with the Leksell stereotactic frame in place, where they had meals and rest.
Twenty-four hours later, the patients received the same radiation dose using the 3-dimensional treatment plan used on the previous day. The Leksell stereotactic frame was then removed and the patients were discharged after simple wound management. For patients with multiple metastasis, additional metastases were treated on the first day using optimal prescription doses.
At the time of the 2-day fraction GKRS, the tumors had a mean volume of 23.1 mL (range, 12.5-67.4). A prescription radiation dose of 10.3 Gy was administered on each day, mainly using a 50% isodose-line. Hence, the fractionated GKRS dose for LBM was 10.3 Gy×2 fractions. All patients underwent serial contrast-enhanced magnetic resonance imaging (MRI) scans every 3 months.
Statistics
Statistical analysis was done on SPSS version 20.0 (IBM Inc., Armonk, NY, USA), with p<0.05 indicating statistically significant differences.
RESULTS
Tumor control and radiation-induced complications
Follow-up clinical and MRI data were obtained for 34 patients (81%) with 35 large brain metastases, who had undergone a 2-day fraction GKRS.
None of the patients exhibited acute or late radiation-induced complications during the follow-up. We investigated the 12 Gy-volume which is known to be related to the radiation-induced complications. The dose of 12 Gy is equivocal dose of 9.3 Gy with two fractions, and they (9.3 Gy×2 volumes) had a median volume with 34.5 mL (range, 21.0-106.0 mL).
Local control failure was indicated by a tumor volume progression of more than 120% based on the most recent MRI. Our analysis revealed a tumor control rate of 48.6% (17 out of 35). The median and mean progression-free survival (PFS) periods were 188 and 194 days, respectively. The local control rates at 6, 9, and 12 months were 77%, 40%, and 34%, respectively. Kaplan-Meier analysis revealed the median PFS to be 235±27 days ( Fig. 1). Kaplan-Meier analysis was used to identify the tumor control prognostic factors associated with meaningful PFS after the 2-day fraction GKRS ( Table 2). This analysis showed that the median PFS of tumors with volumes of ≤20 mL (239±32 days) were similar to those of tumors with volumes of >20 mL (230±32 days, p=0.955). For tumors with volumes of 15 mL, tumor control was not significantly different ( p=0.247). The median PFS period was 199±3 days for tumors with volumes of ≤15 mL and 239±37 days for those with volumes of >15 mL. We additionally investigated if the tumor control was related to the various isodose volumes such as 50%, 70%, and 80% isodose volumes, and the median values were 18.8, 7.50, and 3.3 mL, respectively. PFS of tumors with 50% isodose volume of ≤18.8 mL (230±29 days) were similar to PFS of tumors with volumes of >18.8 mL (239±36 days, p=0.839). Analysis of PFS of tumors with both 70% and 80% isodose volume were all similar to those of 50% isodose volume. PFS of tumors with 70% isodose volume of ≤7.5 mL (238±48 days) were similar to PFS of tumors with volumes of >7.5 mL (235±26 days, p=0.622). PFS of tumors with 80% isodose volume of ≤3.3 mL (239±7 days) were also similar to PFS of tumors with volumes of >3.3 mL (197±32 days, p=0.846).
Among the patients who were followed up, 21 patients underwent peri-GKRS systemic treatment. Most of them underwent cytotoxic chemotherapy (n=19), and the other target agents (n=2). They had total 22 LBMs. In this investigation, ‘peri-GKRS systemic treatment’ was defined as administration of systemic agents at least once during the time of from one month before to months after the 2-day fraction GKRS. The tumor control of the patients with systemic treatment during peri-GKRS periods was similar to that of the patients with no systemic treatments during the same periods. The PFS of the tumors with peri-GKRS systemic treatment was 239±50 days, and the others 230±29 (p=0.741).
Tumors that recurred after previous irradiation exhibited significantly shorter median PFS periods when compared with tumors without prior radiotherapy (85±55 days and 238 ±4 days, respectively; p=0.019). The primary cancer type correlated with tumor control after the 2-day fraction GKRS, and metastasis of lung cancer origin were associated with significantly longer PFS (435±153 days) when compared with other cancers (230± 28 days, p=0.041).
Managing local recurrence and new metastasis after the 2-day fraction GKRS
After the 2-day fraction GKRS, 19 tumors (42.3%) in 19 patients showed local progression and all were local tumor recurrences. None of them were diagnosed with radiation necrosis. To manage these patients, eight underwent a second GKRS, two underwent WBRT, one underwent surgery, and the remaining eight patients received palliative care because of their poor general condition and advanced systemic cancer. After this procedure, new metastases were detected in nine patients. Of these, six underwent a second GKRS, whereas three patients received palliative care because of poor general condition.
Survival after the 2-day fraction GKRS and causes of death
After repeat SRS, the overall survival (OS) of the 44 patients ranged from 15 to 878 days. After the 2-day fraction GKRS, the median and mean OS periods were 263±38 and 174±43 days, respectively. Eight patients (18.2%) were still alive ( Fig. 2). Analysis of the cause of death in 36 patients revealed systemic complications with or without primary cancer progression as the main cause (n=25, 69.4%). Local recurrence following the use of this technique was associated with the death of seven patients (19.4%). Newly developed leptomeningeal metastasis caused the death of one patient (2.8%). However, we could not determine the cause of the deaths of three patients (8.3%).
DISCUSSION
Here, we used 10.3 Gy×2 fractions as the prescription dose. In SRS, based on the dose-effect relationships in the treatment of metastatic brain tumors, higher doses exhibit better tumor control. However, tumor diameter and/or volume is the most important factor in determining the prescription dose. It is generally accepted that the size of the tumor is inversely proportional to the prescription dose. Wiggenraad et al. [ 33] systemically reviewed the dose-effect relationship of SRS on brain metastasis and found that doses of ≤15 Gy were associated with a 12-month local control rate of <50%. We, therefore, considered 16 Gy as the minimum dose that would be expected to have effective local tumor control. Radiation central nervous system toxicity should also be considered. Shaw et al. [ 31] reported that the maximum single fraction radiosurgery doses tolerated by patients with metastatic brain tumors were 24, 18, and 15 Gy for tumors with maximum diameters of ≤20 mm, 21-30 mm, and 31-40 mm, respectively. Based on previous reports and our private experiences, we regarded the minimum effective tumor control dose was as 16 Gy, and the maximum normal brain tolerance dose was 15 Gy at GKRS for LBM. After considering minimizing the number of days for which the stereotactic frame was maintained on the patients, 2 days and one night were chosen. Therefore, we planned the two GKRS fractions so that each dose fraction was delivered at 10.3 Gy. The two-dose (10.3 Gy×2) fractions are equivalent both to single-fraction dose to tumor of 16.05 Gy and to single-fraction dose to brain of 15.12 Gy [ 3, 4, 8, 9]. The efficacy and safety of SRS in the management of metastatic brain tumors are widely accepted, particularly for small-and medium-sized tumors [ 15, 19]. However, SRS is considered to be inappropriate for large metastatic brain tumors because paradoxically, they require lower radiation doses to avoid radiation toxicity, which limits local tumor control [ 15, 31]. To overcome the modest local tumor control achieved in the management of LBM while minimizing SRS-associated radiation toxicity, several novel strategies have been explored. Retrospective and prospective studies have evaluated the use of hypofractionated SRS [ 6, 7, 12, 14, 21- 26, 29, 32, 34] and staged SRS [ 1, 11, 30, 35, 36] to manage LBM, with the aim of increasing the dose to improve local tumor control while minimizing radiation injury [ 1]. However, the hypofractionated SRS studies reported very variable findings on the optimal radiation doses and fractions for each patient based on tumor volume. In this study, we used fixed radiation dose (10.3 Gy) and fractions (two). Kim et al. [ 15] reported the use of GKRS for large (>3 cm) brain metastasis. In that study, they used three fractions and grouped the patients into the 8-, 9- and 10-Gy groups. The patients’ median tumor diameter and volume were 3.6 cm and 15.9 mL, respectively, and the 6- and 12-month actuarial local PFS rates were 87% and 75%, respectively. Based on their findings, they concluded that when compared with doses of 8 Gy×3 or 10 Gy×3 fractions, a regimen of 9 Gy×3 fractions was associated with favorable tumor control and acceptable radiation toxicity. Their analysis of complications revealed that radiation necrosis developed in two patients (12%) in the 9 Gy group, but not in those in the 8 Gy group, which is consistent with our observations. Park et al. [ 27] assessed the use of frameless fractionated GKRS to treat LBM, whereby variable radiation doses (7-10 Gy) and fraction numbers (3-5 fractions) were used. They found that over a mean follow-up of 12 months, the local control rate was 100% and that no patient exhibited radiation necrosis [ 27]. Lim et al. [ 18] investigated the use of fractionated FSRS to treat LBM (with tumor diameters of >3 cm or volumes of >15 mL) and found a 1-year local control rate of 91.7% and an overall complication rate of 17.2%. Navarria et al. [ 25] assessed the use of hypofractionated stereotactic radiotherapy (HSRT) to treat patients with single LBM and found that over a median follow-up of 14 months, local progression at the HSRT site occurred in six patients (5.8 %) and that six patients (5.8%) developed radiation necrosis. We investigated several fractionated SRS methods and converted them into single-fraction equivalent doses to the tumor and the brain ( Table 3). In our study, the local control rates at 6 and 12 months were 77% and 34%, respectively (median PFS, 235±27 days) and these results seem inferior to the above-mentioned recent studies [ 15, 18, 27]. We suspected that one of the reasons related the low tumor control rate and shorter PFS could be the lower prescription dose in our study. The result of no radiation-induced complications in our patients also might be related to the same reason of relatively lower prescription dose. Although a few number of patients and the diversity of primary cancers may be suspected as other suspected reasons, we regarded the prescription dose in this study as the main reason related to the lower tumor control. Our analysis revealed a median PFS of 235±27 days and OS of 263±38 days. Those periods were similar, and the brain-related death rate was only 28.6%. Of the patients who experienced local recurrence after 2-day fraction GKRS, 11 (57.9%) underwent salvage management, such as a second GKRS, WBRT, and surgery. However, the remaining eight patients (42.1%) received palliative care because of advanced systemic cancer.
Although the OS was similar to the PFS, the local control and PFS were unsatisfactory. The deaths of eight patients (15.9%) were associated with local tumor control failure. These observations indicate that this intervention might have been more offensive rather than palliative as a salvage management. We think that a higher prescription dose in this frame-based two fraction setting should be needed. The other efforts to enhance tumor control of LBMs, using various isodoses and different prescription doses to different tumor volumes could be triable techniques. Additionally, we investigated several fractionated SRS methods and converted them into single-fraction equivalent doses to the tumor and the brain ( Table 3).
CONCLUSION
One of the limitation of this investigation was performed for a few numbers of patients with variable primary cancers. The other is, although the result of radiation-induced toxicity was favorable, the tumor control was not satisfactory.
We concluded that a higher prescription dose should be needed in this procedure as a salvage management. Moreover, in the treatment for LBMs with fractionated SRS, using different isodoses and prescription doses at the treatment planning for LBMs could be important. However, we think that this report might be a basic reference with the same fraction number and prescription dose in the treatment for LBMs with frame-based SRS.
Fig. 1.
Progression-free survival of the 35 large volume metastases that underwent 2-day fraction Gamma Knife radiosurgery and follow-up. 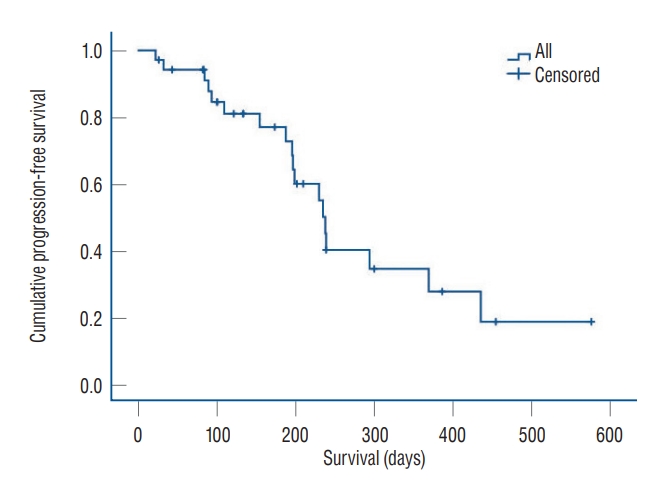
Fig. 2.
Overall survival of the 42 patients with large brain metastasis. 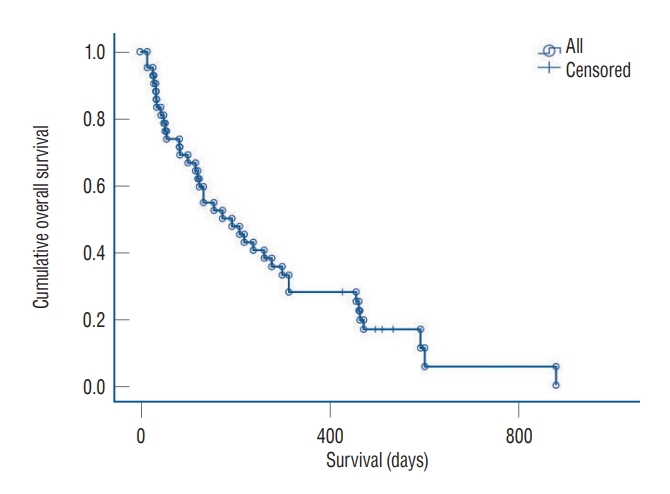
Table 1.
The characteristics of the patients who underwent 2-day fraction GKRS for LBM
|
Characteristic |
Value |
|
Patient |
42 |
|
LBM lesion |
44 |
|
Sex |
|
|
Male |
29 (69.1) |
|
Female |
13 (30.9) |
|
Age (years) |
68.3 (50-84) |
|
Primary cancers |
42 |
|
Non-small cell lung cancer |
16 |
|
Small cell lung cancer |
7 |
|
Colorectal cancer |
7 |
|
Breast cancer |
3 |
|
Gastric cancer |
2 |
|
Ampulla of vater cancer |
1 |
|
Endometrial cancer |
1 |
|
Hepatocellular carcinoma |
1 |
|
Pancreatic cancer |
1 |
|
Pharyngeal cancer |
1 |
|
Renal cell carcinoma |
1 |
|
Small bowel cancer |
1 |
|
LBM and other metastasis/metastases |
42 |
|
One LBM |
21 |
|
One LBM and other metastasis/metastases |
19 |
|
Two LBMs |
2 |
|
Previous treatment for the LBMs |
44 |
|
None |
38 |
|
GKRS |
2 |
|
WBRT |
2 |
|
GKRS and WBRT |
2 |
Table 2.
Univariate analysis of the prognostic factors related to the PFS after 2-day fraction GKRS for large brain metastasis
|
Prognostic factor |
PFS (days) |
p-value |
|
Tumor volume, 20 mL |
|
0.955 |
|
≤20 mL |
239±32 |
|
|
>20 mL |
230±32 |
|
|
Tumor volume, 15 mL |
|
0.247 |
|
≤15 mL |
199±3 |
|
|
>15 mL |
239±37 |
|
|
50% isodose volume |
|
0.839 |
|
≤18.8 mL |
230±29 |
|
|
>18.8 mL |
239±36 |
|
|
70% isodose volume |
|
0.622 |
|
≤7.5 mL |
238±48 |
|
|
>7.5 mL |
235±26 |
|
|
80% isodose volume |
|
0.846 |
|
≤3.3 mL |
239±7 |
|
|
>3.3 mL |
197±32 |
|
|
Peri-GKRS systemic treatment |
|
0.741 |
|
Yes |
239±50 |
|
|
No |
230±29 |
|
|
Previous brain irradiation |
|
0.019 |
|
Yes |
85±55 |
|
|
No |
238±4 |
|
|
Lung cancer primary |
|
0.041 |
|
Yes |
435±153 |
|
|
No |
230±28 |
|
Table 3.
The biologically effective doses of recently reported fractionated stereotactic radiosurgery regimens [ 3, 4, 8, 9]
|
One-day dose×fraction number |
Single-fraction equivalent dose to the tumor |
Single-fraction equivalent dose to the brain |
Reference |
|
10.3 Gy×2 |
16.1 |
15.1 |
Present study |
|
11.6 Gy×2 |
17.9 |
17.0 |
[33] |
|
7 Gy×3 |
14.5 |
13.7 |
[27] |
|
8 Gy×3 |
16.4 |
14.8 |
[15,27,33] |
|
8.5 Gy×3 |
17.3 |
15.7 |
[33] |
|
9 Gy×3 |
18.2 |
16.6 |
[15,22,27] |
|
10 Gy×3 |
20.0 |
18.3 |
[15,27] |
|
8 Gy×5 |
22.3 |
19.5 |
[27] |
References
1. Angelov L, Mohammadi AM, Bennett EE, Abbassy M, Elson P, Chao ST, et al : Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases ≥ 2 cm. J Neurosurg 129 : 366-382, 2018   2. Arvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, et al : Updates in the management of brain metastases. Neuro Oncol 18 : 1043-1065, 2016    3. Barendsen GW : Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys 8 : 1981-1997, 1982   4. Cui T, Weiner J, Danish S, Chundury A, Ohri N, Yue N, et al : Evaluation of biological effective dose in gamma knife staged stereotactic radiosurgery for large brain metastases. Front Oncol 12 : 892139, 2022    5. Eaton BR, LaRiviere MJ, Kim S, Prabhu RS, Patel K, Kandula S, et al : Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neurooncol 123 : 103-111, 2015    6. Feuvret L, Vinchon S, Martin V, Lamproglou I, Halley A, Calugaru V, et al : Stereotactic radiotherapy for large solitary brain metastases. Cancer Radiother 18 : 97-106, 2014   7. Fokas E, Henzel M, Surber G, Kleinert G, Hamm K, Engenhart-Cabillic R : Stereotactic radiosurgery and fractionated stereotactic radiotherapy: comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol 109 : 91-98, 2012    8. Fowler JF : Dose response curves for organ function or cell survival. Br J Radiol 56 : 497-500, 1983   9. Fowler JF : The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 62 : 679-694, 1989    10. Han JH, Kim DG, Chung HT, Paek SH, Park CK, Jung HW : Radiosurgery for large brain metastases. Int J Radiat Oncol Biol Phys 83 : 113-120, 2012   11. Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M, et al : Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 74 : 1543-1548, 2009   13. Jeong WJ, Park JH, Lee EJ, Kim JH, Kim CJ, Cho YH : Efficacy and safety of fractionated stereotactic radiosurgery for large brain metastases. J Korean Neurosurg Soc 58 : 217-224, 2015    15. Kim KH, Kong DS, Cho KR, Lee MH, Choi JW, Seol HJ, et al : Outcome evaluation of patients treated with fractionated gamma knife radiosurgery for large (> 3 cm) brain metastases: a dose-escalation study. J Neurosurg 133 : 675-684, 2019  16. Kirkpatrick JP, Soltys SG, Lo SS, Beal K, Shrieve DC, Brown PD : The radiosurgery fractionation quandary: single fraction or hypofractionation? Neuro Oncol 19( suppl_2):ii38-ii49, 2017    17. Lee CC, Yen CP, Xu Z, Schlesinger D, Sheehan J : Large intracranial metastatic tumors treated by gamma knife surgery: outcomes and prognostic factors. J Neurosurg 120 : 52-59, 2014   18. Lim TK, Kim WK, Yoo CJ, Kim EY, Kim MJ, Yee GT : Fractionated stereotactic radiosurgery for brain metastases using the Novalis Tx ® system. J Korean Neurosurg Soc 61 : 525-529, 2018     19. Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R : Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev 40 : 48-59, 2014   20. Luther N, Kondziolka D, Kano H, Mousavi SH, Engh JA, Niranjan A, et al : Predicting tumor control after resection bed radiosurgery of brain metastases. Neurosurgery 73 : 1001-1006; discussion 1006, 2013    21. Minniti G, D’Angelillo RM, Scaringi C, Trodella LE, Clarke E, Matteucci P, et al : Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol 117 : 295-301, 2014    22. Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, et al : Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95 : 1142-1148, 2016   23. Murai T, Ogino H, Manabe Y, Iwabuchi M, Okumura T, Matsushita Y, et al : Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases: a dose escalation study. Clin Oncol (R Coll Radiol) 26 : 151-158, 2014   24. Narayana A, Chang J, Yenice K, Chan K, Lymberis S, Brennan C, et al : Hypofractionated stereotactic radiotherapy using intensity-modulated radiotherapy in patients with one or two brain metastases. Stereotact Funct Neurosurg 85 : 82-87, 2007    25. Navarria P, Pessina F, Cozzi L, Ascolese AM, De Rose F, Fogliata A, et al : Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol 11 : 76, 2016    26. Ogura K, Mizowaki T, Ogura M, Sakanaka K, Arakawa Y, Miyamoto S, et al : Outcomes of hypofractionated stereotactic radiotherapy for metastatic brain tumors with high risk factors. J Neurooncol 109 : 425-432, 2012    28. Pessina F, Navarria P, Cozzi L, Ascolese AM, Maggi G, Rossi M, et al : Role of surgical resection in patients with single large brain metastases: feasibility, morbidity, and local control evaluation. World Neurosurg 94 : 6-12, 2016   29. Redmond KJ, Gui C, Benedict S, Milano MT, Grimm J, Vargo JA, et al : Tumor control probability of radiosurgery and fractionated stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 110 : 53-67, 2021   30. Serizawa T, Higuchi Y, Yamamoto M, Matsunaga S, Nagano O, Sato Y, et al : Comparison of treatment results between 3- and 2-stage gamma knife radiosurgery for large brain metastases: a retrospective multiinstitutional study. J Neurosurg 131 : 227-237, 2018   31. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al : Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47 : 291-298, 2000   32. Wegner RE, Leeman JE, Kabolizadeh P, Rwigema JC, Mintz AH, Burton SA, et al : Fractionated stereotactic radiosurgery for large brain metastases. Am J Clin Oncol 38 : 135-139, 2015   33. Wiggenraad R, Verbeek-de Kanter A, Kal HB, Taphoorn M, Vissers T, Struikmans H : Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol 98 : 292-297, 2011   34. Wiggenraad R, Verbeek-de Kanter A, Mast M, Molenaar R, Kal HB, Lycklama à Nijeholt G, et al : Local progression and pseudo progression after single fraction or fractionated stereotactic radiotherapy for large brain metastases. A single centre study. Strahlenther Onkol 188 : 696-701, 2012    36. Yomo S, Hayashi M, Nicholson C : A prospective pilot study of two-session gamma knife surgery for large metastatic brain tumors. J Neurooncol 109 : 159-165, 2012    37. Zimmerman AL, Murphy ES, Suh JH, Vogelbaum MA, Barnett GH, Angelov L, et al : Treatment of large brain metastases with stereotactic radiosurgery. Technol Cancer Res Treat 15 : 186-195, 2016   
|
|


















