Kang: Update on the Vein of Galen Aneurysmal Malformation : Disease Concept and Genetics
Abstract
Vein of Galen aneurysmal malformation is one of important pediatric arteriovenous shunt diseases, especially among neonates and infants. Here, early history of the disease identification, basic pathoanatomy with a focus on the embryonic median prosencephalic vein, classification and differential diagnoses, and recent genetic studies are reviewed.
Key Words: Vein of Galen malformations · Arteriovenous fistula · Pediatrics · Genetic testing.
EARLY HISTORY
Vein of Galen aneurysmal malformations (VGAMs) can be defined as direct arteriovenous fistulas (AVFs) between choroidal and/or quadrigeminal arteries and an overlying single median venous sac, which is the persistence of the embryonic median prosencephalic vein (MPV) of Markowski [ 25]. The latter drains the choroid plexuses of the lateral and third ventricles between the 7th and 12th weeks of gestation, and disappears normally to be replaced by the internal cerebral veins (ICVs), when the intrinsic vascularization of the neural tube develops [ 25]. Lasjaunias wrote that ‘the first description of a possible VGAM occurred in 1895 (Steinheil, cited by Dandy in 19287)).’ in his book [ 21]. This has been cited in a number of following publications. However, we can easily find that was actually a case of basal frontal arteriovenous malformation (AVM) with a possible secondary dilatation of the vein of Galen (see Fig. 32F in the article) [ 7]. A case of an aneurysm of the vein of Galen was described as early as 1937 by Jaeger [ 14]. The authors added a detailed description of the case 9 years later [ 15]. The latter work was stimulated by additional case reports between 1940 and 1945 [ 1, 28]. All the drawings from necropsy were made by Mrs. Padget, and there is ‘tortuous vessels interposed’ (see Fig. 4 in the article) which can be an arterioarterial maze in the choroidal type of VGAM or a nidus of thalamic AVM [ 15]. Boldrey and Miller [ 4] performed angiography and carotid ligation (plus clipping of the posterior cerebral artery in one case) for two patients of VGAM. This seems to the first report of angiographic evaluation for the VGAM. The unsubtracted angiograms here demonstrate well the dilated feeders and aneurysmal dilation of the vein of Galen. Oscherwitz and Davidoff [ 24] described a case with midline calcified round mass, for which the diagnosis seems to be controversial. These early descriptions related to VGAM are summarized in Table 1.
MPV
The MPV, also known as vena mediana prosencephali or the primitive ICV ‘for convenience’, in human is an embryonic vein that appears as early as 32 days of gestation (8 to 11 mm embryo) and disappears at 11th week (50 mm embryo), as is reviewed by Raybaud et al. [ 25]. They cite early works by Mall (1904-1905), Streeter (1918), Hochstetter (1938), Ariens Kappers (1955), and Padget (1957). This single midline temporary vein is distinctly different from the permanent paired ICVs. The MPV is the first vein to drain an intracerebral structure because the choroid plexus develops before the neural parenchyma has been penetrated by vessels. Progression of intracerebral vascularization and development of basal ganglia result in the formation of the paired ICVs which soon annex the venous drainage of the choroid plexus. This change leads to regression and disappearance of MPV, except for its most caudal portion which joins ICVs to form the vein of Galen. Thus, Raybaud et al. [ 25] was the first to recognize that the ectatic vein in VGAM is the MPV, the embryonic precursor of the vein of Galen. Otherwise stated, the single median venous sac or ‘ampulla’, as what we see and as is mentioned previously [ 12], is not the vein of Galen per se, but the persistence of the embryonic MPV. This concept is well illustrated already [ 21]. The embryonic MPV primarily drains the choroidal afferents and secondarily collects the lenticulostriate (thalamostriate) afferents. Eventually the normal vein of Galen becomes the deep venous confluent opening into the straight sinus. In patients with VGAM, there is an arteriovenous shunt and the MPV persists and bulges. Here the choroidal vein and the thalamostriate vein drain separately, rather than into the vein of Galen, making the so called ‘epsilon configuration’ composed of thalamic and subtemporal veins [ 21]. Thus, there has been a notion that the deep venous system is not connected to and does not drain into the ectatic MPV and vein of Galen in children with a VGAM. However, attention should be paid to the possible connection of the venous pouch to the deep veins including the ICV [ 10, 23, 25]. In the series of Raybaud et al. [ 25] one ICV was connected to the aneurysmal sac in six of 12 cases, without its dilatation. No connection was observed between the aneurysm and the basal vein or the precentral cerebellar vein in this study. In other reports, the connection between ICV and the aneurysmal vein was demonstrated with selective retrograde transvenous microcatheterization [ 23] or post-treatment control MR imaging [ 10]. In an adult case of VGAM, the connection to the basal vein was also demonstrated, in addition to bilateral ICVs [ 23]. The possible presence of such deep venous drainage need to be kept in mind to avoid disastrous adverse effects, i.e., venous infarction and/or intracranial hemorrhage, when the malformation is occluded on the venous side. Selective obliteration at the site of fistula should be preferred to complete occlusion of the venous pouch, as is well illustrated previously [ 23]. Pre-treatment angiography may not guarantee the absence or presence of deep venous connection to the shunt lesion since such factors as high-velocity shunt, preferential flow and elevated venous pressure would impede a nice demonstration of the real anatomical situations.
CLASSIFICATION AND DIFFERENTIAL DIAGNOSES
Johnston et al. [ 16] defined VGAM as ‘an aneurysmal dilatation of the vein of Galen that has an arterial input from one or more of the major intracranial arteries either directly or via an interposed angiomatous malformation.’ Therefore, the authors acknowledged there are (at least) two different kinds of the disease. One has a direct shunt and the other has an interposed angiomatous malformation. Raybaud et al. [ 25] classified the pattern of arteriovenous communication as single, multiple, and interposed arterioarterial maze, which we really encounter in clinical practice. Superselective angiography would reveal the angioarchitecture precisely. In their analysis, the posterior choroidal arteries were the most common feeders, follow by the anterior cerebral artery, explaining that the distal posterior pericallosal branch of the anterior cerebral artery is an embryonic choroidal artery [ 25]. According to them the anterior cerebral artery normally serves the choroid plexus at the interventricular foramen by way of a posterior branch curving around the splenium, and this connection is constant in the embryo. Thus, the embryonic limbic arterial arch should be the principal feeders to VGAM [ 3, 21]. In 1989, Lasjaunias et al. [ 20] classified their 36 cases of vascular lesions in the vein of Galen region into five types : 1) true (or mural) VGAM, 2) choroidal fissure AVF, 3) parenchymatous AVM with vein of Galen dilatation, 4) dural vein of Galen fistula, and 5) vein of Galen varix. Later, Lasjaunias et al. [ 21] included only two types, choroidal and mural, in the chapter of VGAM, excluding the others. The nidus (or shunted pouch) of the lesion is usually located in the midline, and one side may be more prominent in certain cases where the dilated pouch is shifted by the force of jet of the fistula away from the prominent supply toward the opposite side [ 21]. Exemplary cases are shown in Fig. 1. The choroidal type corresponds to a very primitive condition, with the contribution of all the choroidal arteries and an interposed network before opening into the large venous pouch [ 21]. The interposed network in the choroidal type VGAM has been described as an interposed angiomatous malformation [ 16] or as interposed arterioarterial maze [ 25], which should be differentiated from AVM nidus. As shown previously [ 20], VGAM can be confused with tectal, third ventricle, pineal, fornix, thalamic, mesencephalic or posterior cingular AVM. The mural type corresponds to direct AVF within the wall of the MPV [ 21]. The fistula can be single or, more often, multiple. The latter can converge into a single venous chamber or into multiple venous lobulations along the anterior aspect of the pouch or along the choroidal veins of the fissure [ 19]. This mural form is often better tolerated and encountered in infants with better disease tolerance and no cardiac symptoms. Intermediate forms or mixed forms can occur. Yasargil’s classification scheme provides information about the vascular anatomy of the lesions [ 33]. Type I is a pure cisternal fistula between the vein of Galen and either the pericallosal or posterior cerebral arteries, which seems to be most similar to the mural type VGAM in Lasjaunias’ classification scheme. Type II has multiple fistulous communications between the vein of Galen and the thalamoperforating vessels. Type III is high-flow mixed from of type I and type II. This type is comparable to the choroidal type VGAM, although there is no description of interposed angiomatous network. Type IV is a parenchymal AVM with drainage into the vein of Galen, which is not a true VGAM. Some authors call type IV lesion as vein of Galen aneurysmal dilatation or, in short, VGAD, as described later. As suggested by Lasjaunias et al. [ 21], VGAM needs to be differentiated from other conditions related to an enlarged vein of Galen. One of these is VGAD, which represents a group of cerebral AVMs draining into the deep venous system with ectatic dilatation of the vein of Galen confluence due to either stenosis at the venodural junction or thrombosis of the straight sinus. This corresponds to type IV lesion in Yasargil’s classification. Vein of Galen varix is dilatation of the vein without the presence of an arteriovenous shunt [ 21]. One type of this group includes transient dilatation of the vein in neonates presenting with heart failure of another origin. The second type of vein of Galen varix occurs when the hemispheric venous drainage converges toward the deep venous system, which corresponds to a type of developmental venous anomaly. Finally, dural arteriovenous shunt with vein of Galen dilatation should be differentiated from VGAM [ 21]. This is usually seen in adults.
GENETICS
The genetic understanding of VGAM has been hindered by its rarity and sporadic nature [ 34]. In 2008, Revencu et al. [ 27] studied on 140 patients with RAS P21 protein activator 1 gene ( RASA1) mutation and found two with VGAM. Others had capillary malformation (CM) (n=134), other AVM or AVF (n=24), Parkes Weber syndrome (n=17). The gene RASA1 is an NF1 homolog and the protein encoded by RASA1, p120RasGAP, is an inhibitor of RAS p21. Hence, impaired RASA1 activity leads to RAS remaining locked in a GTP-bound configuration, thereby leading to constitutive RAS activation. In 2011, Tsutsumi et al. [ 30] reported a child with VGAM had a mutation in endoglin ( ENG) gene. His mother had hereditary hemorrhagic telangiectasia (HHT). In 2013, Heuchan et al. [ 13] found RASA1 mutations in four of 11 patients with VGAM. In the same year, Chida et al. [ 6] screened for mutations in RASA1 and three HHT genes ( ENG, activin A receptor like type 1 [ ACVRL1], SMAD4) in four VGAM patients and found a variant in ACVRL1, encoding ALK1, in a patient. ALK1 is a member of the bone morphogenetic protein family that belongs to the transforming growth factor-beta (TGF-β) superfamily. Duran et al. [ 8] underwent exome sequencing of 55 VGAM probands, including 52 parent-offspring trios, and demonstrated enrichment of damaging de novo mutations in chromatin modifier genes (histone-lysine N-methyltransferase 2D [ KMT2D], SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 2 [ SMARCA2], silent mating type information regulation 2 homologue [sirtuin] 1 [ SIRT1], and lysine acetyltransferase 6A [ KAT6A]). They suggest that neurodevelopmental phenotypes in VGAM patients currently attributed to secondary damage to the central nervous system may, instead, reflect primary impairment from genetic mutation. In addition, other probands harbored inherited damaging mutations in ephrin signaling genes, ephrin B2 ( EFNB2) and ephrin receptor-B4 ( EPHB4). The roles of EFNB2 and EphB4 in arteriovenous specification are well established since two pivotal studies published in 1998 and 1999 [ 11, 31]. Targeted disruption of the EFNB2 gene prevents the remodeling of veins from a capillary plexus into properly branched structures and also hinders the remodeling of arteries [ 31]. A targeted mutation in EPHB4 essentially phenocopies the mutation in EFNB2 [ 11]. The mutant phenotypes in both the EFNB2-/- and EPHB4-/- capillary plexuses extended beyond the arteriovenous boundary. Heterozygous EPHB4 germline mutations contribute to a spectrum of vascular pathology and EPHB4 is believed to be a bona fide VGAM risk gene. Zhao et al. [ 34] performed an integrated analysis of 310 VGAM proband-family exomes and 336326 human cerebrovasculature single-cell transcriptomes, and found important damaging variants related to VGAM development. They included RASA1, EPHB4, ACVRL1, notch receptor 1 [ NOTCH1], ITGB1, and protein tyrosine phosphatase non-receptor type 11 ( PTPN11). In this study, developing endothelial cells was defined as a likely spatio-temporal locus of VGAM pathophysiology. They noted that genetic dysregulation of Ras signaling is an important driver of VGAM pathogenesis based on the following : 1) RASA1 and EPHB4 cooperate to regulate Ras signaling in endothelial cells, 2) PTPN11 binds to and dephosphorylates Ras to increase its association with Raf and activate Ras signaling [ 9], and 3) ACVRL1 facilitates crosstalk between the TGF-β and Ras signaling pathways by associating with RASA1 via the Dok1 adapter protein [ 32]. Those genes already have been reported in other Mendelian vascular diseases and it is plausible that VGAM may represent another phenotypic expansion of CM-AVM, HHT, or other Mendelian vascular syndromes [ 34]. RASA1 and EPHB4 encode interacting proteins mutated in CM-AVM type 1 and 2, respectively [ 2, 26]. ACVRL1 (ALK1) is linked with HHT2, and mutations of HHT1 gene ENG, encoding the ALK1 binding partner Endoglin, are reported in association with VGAM [ 29, 30]. In a report, a consanguineous couple had recurrent VGAM in two pregnancies. And both partners were affected by HHT due to a known pathogenic heterozygous c.790G>A (p.Asp264Asn) variant in ENG [ 29]. Additional variant in NOTCH genes, NOTCH3 and NOTCH4, were found in a series of VGAM neonates through next-generation sequencing [ 5]. In a neonate, there was a heterozygous c.2903C>A (p.Ser968Ter) variant in the NOTCH3 gene, which introduced the premature stop codon. This was classified as a pathogenic variant (class 5) according to the American College of Medical Genetics and Genomics (ACMG) guidelines. In the other, there was a heterozygous c.4855C>A (p.Leu1619Met) variant in the NOTCH4 gene, leading to the amino acid change. This was classified as a variant of unknown significance (ACMG class 3). Both of the patients presented with choroidal type VGAMs. Activation of the Notch pathway suppresses venous cell fate and its impairment results in arteriovenous shunt in zebrafish [ 22]. CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) is a well-established disease characterized by early adult-onset stroke and dementia, is caused by mutations in NOTCH3 [ 17]. NOTCH signaling has an important role in vessel homeostasis and in regulating vascular smooth muscle cell differentiation [ 18]. The studies on the genetics related to VGAM are summarized in Table 2. Further studies on the VGAM are required to deepen understanding of the disease and to identify potential druggable target.
Fig. 1.
Exemplary cases of vein of Galen aneurysmal malformation (VGAM). A and B : The left vertebral angiograms show a choroidal type of VGAM in a 2-week-old baby boy presenting with heart failure. The malformation has multiple feeders from the posterior choroidal arteries bilaterally and the posterior thalamoperforator, and is drained to the malformed vein (which is the persistent median prosencephalic vein) and then into the falcine sinus. C and D : The left vertebral angiograms show a mural type of VGAM in a 2-year-old boy presenting with macrocrania. There is a single-hole shunt (arrow) from the right posterior choroidal arteries. 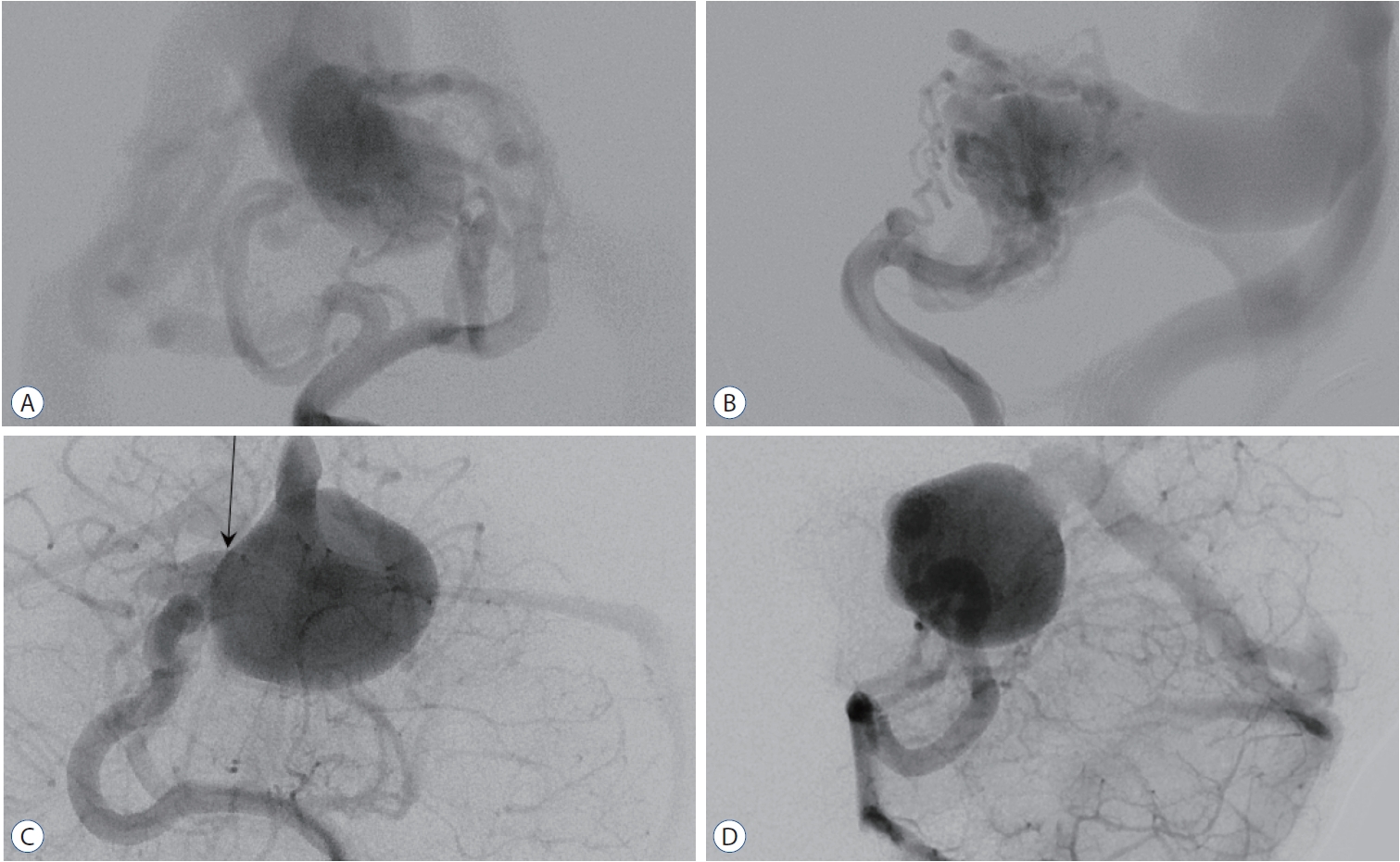
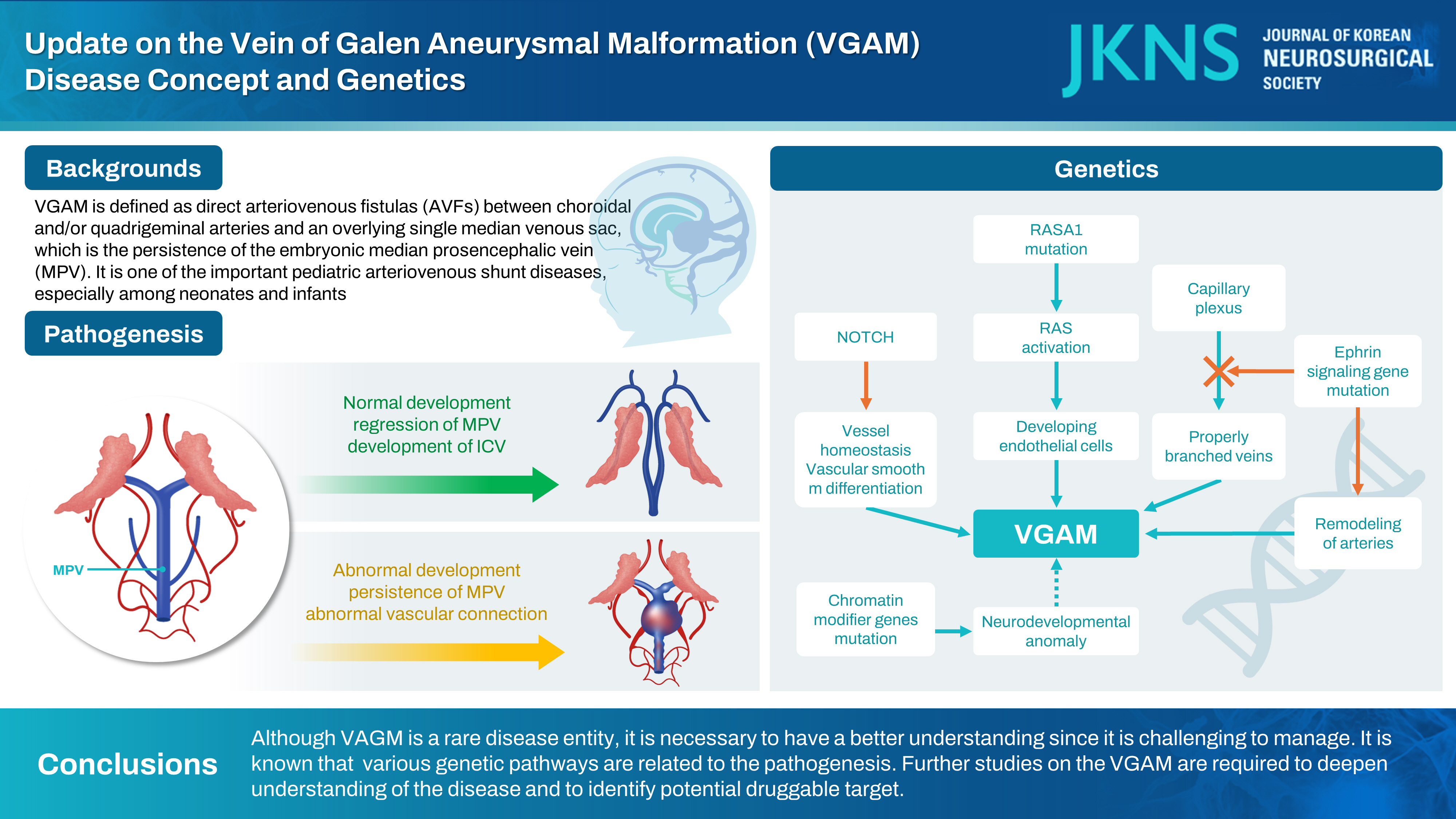
Table 1.
Early description of possible VGAM cases
|
Study |
Sex & age |
Diagnosis |
|
Steinheil (1895) (cited in Dandy [7] [1928]) |
NA |
Parenchymal AVM with a secondary dilatation of the vein of Galen |
|
Jaeger [14] (1937), Jaeger and Forbes [15] (1946) |
Male, 15 months to 4 years |
VGAM likely at necropsy |
|
Russell and Nevin [28] (1940) |
Male, 17 months |
VGAM likely at necropsy |
|
Russell and Nevin [28] (1940) |
Male, 17 months |
VGAM likely at necropsy |
|
Alpers and Forster [1] (1945) |
Male, 18 years |
VGAM likely at necropsy |
|
Oscherwitz and Davidoff [24] (1947) |
Female, 27 years |
Round calcified mass at the pineal region with an uncertain diagnosis |
|
Boldrey and Miller [4] (1949) |
Male, 16 months |
VGAM at angiography; carotid ligation and posterior cerebral artery clipping |
|
Boldrey and Miller [4] (1949) |
Male, 15 years |
VGAM at angiography; carotid ligation |
Table 2.
Genes related to VGAM development
|
Study |
Gene |
|
Revencu et al. [27] (2008) |
RASA1
|
|
Tsutsumi et al. [30] (2011) |
Endoglin |
|
Heuchan et al. [13] (2013) |
RASA1
|
|
Chida et al. [6] (2013) |
ACVRL1
|
|
Duran et al. [8] (2019) |
KEL, KMT2D, SMARCA2, SIRT1, KAT6A, EPHB4, CLDN14, EFNB2, RASA1, ACVRL1, ACVR1
|
|
Singh et al. [29] (2022) |
Endoglin |
|
Campi et al. [5] (2023) |
NOTCH3, NOTCH4
|
|
Zhao et al. [34] (2023) |
RASA1, EPHB4, ACVRL1, NOTCH1, ITGB2, PTPN11
|
References
1. Alpers BJ, Forster FM : Arteriovenous aneurysm of great cerebral vein and arteries of circle of Willis; formation by junction of the great cerebral vein and the straight sinus and by the choroidal arteries and anomalous branches of the posterior cerebral arteries. Arch Neurol Psychiatry 54 : 181-185, 1945  2. Amyere M, Revencu N, Helaers R, Pairet E, Baselga E, Cordisco M, et al : Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation 136 : 1037-1048, 2017   3. Bhattacharya JJ, Thammaroj J : Vein of Galen malformations. J Neurol Neurosurg Psychiatry 74( Suppl 1):i42-i44, 2003    4. Boldrey E, Miller ER : Arteriovenous fistula (aneurysm) of the great cerebral vein (of Galen) and the circle of Willis; report on two patients treated by ligation. Arch Neurol Psychiatry 62 : 778-783; illust, 1949  5. Campi F, De Rose DU, Pugnaloni F, Ronci S, Calì M, Pro S, et al : Neurodevelopmental and genetic findings in neonates with intracranial arteriovenous shunts: a case series. Front Pediatr 11 : 1111527, 2023    6. Chida A, Shintani M, Wakamatsu H, Tsutsumi Y, Iizuka Y, Kawaguchi N, et al : ACVRL1 gene variant in a patient with vein of Galen aneurysmal malformation. J Pediatr Genet 2 : 181-189, 2013   7. Dandy WE : Arteriovenous aneurysm of the brain. Arch Surg 17 : 190-243, 1928  8. Duran D, Zeng X, Jin SC, Choi J, Nelson-Williams C, Yatsula B, et al : Mutations in chromatin modifier and ephrin signaling genes in vein of Galen malformation. Neuron 101 : 429-443.e4, 2019   9. Fattah M, Raman MM, Reiss AL, Green T : PTPN11 mutations in the RasMAPK signaling pathway affect human white matter microstructure. Cereb Cortex 31 : 1489-1499, 2021    10. Gailloud P, O’Riordan DP, Burger I, Lehmann CU : Confirmation of communication between deep venous drainage and the vein of Galen after treatment of a vein of Galen aneurysmal malformation in an infant presenting with severe pulmonary hypertension. AJNR Am J Neuroradiol 27 : 317-320, 2006   11. Gerety SS, Wang HU, Chen ZF, Anderson DJ : Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell 4 : 403-414, 1999   12. Hamilton MG, Herman JM, Khyata MH, Spetzler RF : Aneurysms of the vein of Galen in Youmans JR (eds) : Youmans Neurological Surgery, ed 4. Philadelphia : W.B. Saunders, 1996, Vol 2 : pp1491-1510
13. Heuchan AM, Joss S, Berg J, Suri M, Bhattacharya J : G25 RASA1 mutations and vein of Galen arterial malformation. Arch Dis Child 98(Suppl 1):A16-A17, 2013
14. Jaeger JR : Bilateral congenital cerebral arteriovenous communication aneurysm. Trans Am Neurol Assoc 63 : 173-176, 1937
15. Jaeger R, Forbes RP : Bilateral congenital arteriovenous communications (aneurysm) of the cerebral vessels. Arch Neurol Psychiatry 55 : 591-599, 1946   16. Johnston IH, Whittle IR, Besser M, Morgan MK : Vein of Galen malformation: diagnosis and management. Neurosurgery 20 : 747-758, 1987   17. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al : Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383 : 707-710, 1996    20. Lasjaunias P, Rodesch G, Terbrugge K, Pruvost P, Devictor D, Comoy J, et al : Vein of Galen aneurysmal malformations. Report of 36 cases managed between 1982 and 1988. Acta Neurochir (Wien) 99 : 26-37, 1989  21. Lasjaunias P, ter Brugge KG, Berenstein A : Surgical Neuroangiography, ed 2. Berlin : Springer-Verlag, 2006, Vol 3 : pp105-226
22. Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, et al : Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128 : 3675-3683, 2001    23. Levrier O, Gailloud PH, Souei M, Manera L, Brunel H, Raybaud C : Normal galenic drainage of the deep cerebral venous system in two cases of vein of Galen aneurysmal malformation. Childs Nerv Syst 20 : 91-97; discussion 98-99, 2004    24. Oscherwitz D, Davidoff LM : Midline calcified intracranial aneurysm between occipital lobes; report of a case. J Neurosurg 4 : 539-541, 1947  25. Raybaud CA, Strother CM, Hald JK : Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology 31 : 109-128, 1989    26. Revencu N, Boon LM, Mendola A, Cordisco MR, Dubois J, Clapuyt P, et al : RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum Mutat 34 : 1632-1641, 2013  27. Revencu N, Boon LM, Mulliken JB, Enjolras O, Cordisco MR, Burrows PE, et al : Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat 29 : 959-965, 2008   28. Russell DS, Nevin S : Aneurysm of the great vein of Galen causing internal hydrocephalus: report of two cases. J Path Bact 51 : 375-383, 1940  30. Tsutsumi Y, Kosaki R, Itoh Y, Tsukamoto K, Matsuoka R, Shintani M, et al : Vein of Galen aneurysmal malformation associated with an endoglin gene mutation. Pediatrics 128 : e1307-e1310, 2011    31. Wang HU, Chen ZF, Anderson DJ : Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93 : 741-753, 1998   32. Yamakawa N, Tsuchida K, Sugino H : The rasGAP-binding protein, Dok1, mediates activin signaling via serine/threonine kinase receptors. EMBO J 21 : 1684-1694, 2002    33. Yasargil MG : Microneurosurgery, ed 1. New York : Thieme, 1988, Vol IIIB : pp323-357
34. Zhao S, Mekbib KY, van der Ent MA, Allington G, Prendergast A, Chau JE, et al : Mutation of key signaling regulators of cerebrovascular development in vein of Galen malformations. Nat Commun 14 : 7452, 2023  
|
|


















