Cheon and Kim: Congenital Intracranial Vascular Malformations in Children : Radiological Overview
Abstract
Prompt medical attention is crucial for congenital intracranial vascular malformations in children and newborns due to potential severe outcomes. Imaging is pivotal for accurate identification, given the diverse risks and treatment strategies. This article aims to enhance the identification and understanding of congenital intracranial vascular abnormalities including arteriovenous malformation, arteriovenous fistula, cavernous malformation, capillary telangiectasia, developmental venous anomaly, and sinus pericranii in pediatric patients.
Key Words: Central nervous system vascular malformations · Intracranial arteriovenous malformations · Arteriovenous fistula · Diagnostic imaging · Congenital.
INTRODUCTION
Congenital intracranial vascular malformation in children and newborns, though exceedingly rare, require immediate medical attention due to their potential for severe consequences. The spectrum of cerebral vascular malformation in children include cerebral arteriovenous malformations (AVMs), arteriovenous fistulas (AVFs), vein of Galen aneurysmal malformation (VGAM), cavernous malformation (CM), capillary telangiectasia, developmental venous anomaly (DVA) and sinus pericranii. Identifying these malformations through imaging is crucial, given the varying risks and treatment strategies associate with each [ 15, 23]. For example, intracranial vascular malformations with arteriovenous shunting, i.e., classic brain AVMs, VGAM or pial AVFs are potentially amendable to endovascular intervention. Conversely, intracranial vascular malformation without arteriovenous shunting, i.e., CM, DVAs or capillary telangiectasia are either treated surgically or left alone [ 21]. In this article, we present a practical diagnostic approach based on imaging for suspected vascular lesions of the brain. Through this exploration, we aim to provide valuable insights into the identification and understanding of congenital intracranial vascular abnormalities in the brain, facilitating more informed and effective medical decision-making.
INTRACRANIAL VASCULAR MALFORMATIONS WITH ARTERIOVENOUS SHUNTS
AVMs
AVMs are abnormal vascular connection within the brain characterized by high-flow arteriovenous shunting through a complex nidus of arterioles and venules. The overall incidence of brain AVMs has been reported ranging from 1.10 to 1.42 cases per 100000 people [ 3]. AVMs are considered as the most common cause of spontaneous intracranial hemorrhage in children. Other symptoms in children include headache, seizures, or other neurological symptoms. When AVMs rupture, they contribute to a significant higher percentage of spontaneous intracranial hemorrhage in pediatric patients compare to adults, accounting for 30-50% vs. 1.4-2%, respectively. For untreated AVMs in children, there is an annual rupture risk of 4.4-5.5%, which is substantially reduced after treatment [ 4, 11]. This reduction underscores the importance of early intervention in managing pediatric intracranial AVMs. Two subtypes of abnormal vessel networks can be encountered when a nidus is present. The typical type is the glomerular or compact type nidus, consisting of abnormal vessels without any interspersed normal brain tissue. Best diagnostic clue of AVM is parenchymal hemorrhage and the presence of a cluster of adjacent abnormal vessels forming the nidus with enlarged draining veins. An AVM shows a densely packed mass of enlarged vascular channels with noticeable flow voids on magnetic resonance imaging (MRI). Susceptibility-weighted image (SWI) or gradient recalled echo (GRE) sequences show blooming indicative of hemorrhagic blood products. Hyperintense SWI signals in draining veins reflect rapid arteriovenous shunting. Time-resolved MR angiography is effective in visualizing arteriovenous shunting. Avid enhancement of nidus and draining veins can be seen on GRE T1-weighted image (not spin echo images). Digital subtraction angiography (DSA) remains the gold standard for evaluating the arterial feeding vessels, distinguishing between deep and superficial draining veins, and identifying associated feeding artery aneurysms (10-15%) or intranidal aneurysms (approximately 50%), as well as venous stenoses (35-60%). This detailed assessment with DSA is crucial for determining the optimal treatment strategy [ 5, 13, 26] ( Fig. 1). The more rarely seen second type of AVM is the so-called cerebral proliferative angiopathy (CPA) characterized by diffuse or proliferative type of nidus and the interspersion of normal brain parenchyma within a tangle of vessels. CPA, previously identified as the diffuse nidus type AVM, accounts for approximately 2-4% of all brain AVMs. It is recognized as a distinct entity from the classic brain AVM and exhibits a female predilection (2 : 1) with a relatively young mean patient age of 20 years [ 9, 14, 19, 23]. Abnormal vessels intertwining with normal brain parenchyma are characteristic features of CPA on brain MRI or computed tomography (CT). Frequently, an entire lobe or even a brain hemisphere is affected. DSA reveals arterial feeder vessels of normal size or only moderately enlarged. Associated stenoses of the feeder vessels are often detected, with extensive transdural supply to both normal and abnormal brain tissue through branches of the external carotid artery. Crucially, the absence of clear early venous drainage on dynamic images is pivotal for differentiating CPA from classic brain AVMs [ 5] ( Fig. 2). When confronted with this finding, it becomes imperative to consider CPA or cerebrofacial arteriovenous metameric syndrome in the differential diagnosis. Cerebrofacial arteriovenous metameric syndrome is characterized by the presence of multiple AVMs in both the brain parenchyma and the facial region.
Congenital AVFs
AVFs are rare vascular anomalies in children, but they are relatively more common within this age group compared to other vascular lesions [ 21]. The prevalence of AVFs ranges between 0.1/100000 and 1/100000, with no discernible sex predilection. AVFs comprise approximately 4% of pediatric cerebral vascular malformations [ 15]. These lesions can be categorized into three entities : VGAM, pial AVF, and dural AVF within the context of dural sinus malformations (DSMs) [ 7, 8, 12].
VGAM
VGAM is a rare subtype of a dural AVF occurring between deep choroidal arteries and median prosencephalic vein (MPV) is typically diagnosed antenatally and may present more severely in infancy and it is the most common extracardiac cause of high-output hear failure in newborns. A vein of Galen is not formed, and the venous drainage is toward its precursor, the MPV and subsequently through a persistent falcine sinus. There are two types of VGMs, the choroidal and the mural types. The choroidal type represents a more primitive condition featuring an intervening network between the multiple primitive choroidal arteries and the venous pouch before entering MPV ( Fig. 3). The mural type corresponds to direct AV fistulas within the wall of MPV ( Fig. 4) [ 1, 8, 20]. A two-dimensional real-time ultrasonography can identify an aneurysm as a hypoechoic midline mass posterior to the roof of the third ventricle. The use of color Doppler ultrasound provides insight into hemodynamics including increased middle cerebral artery (MCA) pressure, high turbulence, and bidirectional blood flow within the aneurysm. Ultrasonography aids in identifying associated anomalies such as hydrocephalus. Postnatal follow-up of endovascularly treated VGAM can be monitored using ultrasonography.
MRI has become the preferred imaging modality for VGAM. It accurately visualizes hydrocephalus, cortical atrophy, and injury of white matter, helping differentiate a VGAM from a cerebral AVM that drains into the vein of Galen. MR angiography serves as a noninvasive alternative for the initial evaluation of a suspected VGAM. Both MRI and MR angiography play crucial roles in understanding the lesion prior to and at the time of endovascular embolization, respectively. DSA allows for easier visualization of small arteries, which feed into the fistulas, and it also shows the relationship between the arteriovenous shunt and the venous drainage.
Pial AVF
Pial AVFs are congenital high-flow arteriovenous shunts between pial or cortical arteries and a single dilated and tortuous vein. Varix formation is a unique finding in almost all patients with pial AVF ( Fig. 5). In contrast to AVM, pial AVFs lack an interposed nidus between the arterial and venous sides. Additionally, pial AVFs are in the subpial space rather than in the dural leaflets, distinguishing them from dural AVF. Newborns typically exhibit congenital heart failure as the primary clinical presentation due to shunt overload, while older children are more prone to intracranial hemorrhage and seizures. Pial AVFs are frequently associated with hemorrhagic telangiectasia. Clues to the diagnosis of pial AVFs on cross-sectional imaging include the presence of dilated vessels, mainly at the brain surface and asymmetric dilatation of the pial feeding artery, either the MCA, anterior cerebral artery, or posterior cerebral artery, at the level of the circle of Willis ( Fig. 5). These imaging findings can be used to differentiate pial AVFs from dural AVFs and may be accompanied by dilated venous pouches outside the brain parenchyma [ 2, 7].
Dural AVF
Intracranial dural AVF is a form of cerebral vascular malformation characterized by a shunt between extracranial vessels and venules in the wall of dural venous sinus. Infantile or juvenile type dural AVFs are high-flow low pressure lesions with multiple arteriovenous shunts draining into an enlarged dural sinus. Dural AVFs are sometimes associated with DSMs. DSMs are congenital and can be detected either antenatally, in neonates or infants and characterized by enlargement of the affected dural sinuses, most commonly the torcular or superior sagittal sinus. DSM without arteriovenous shunts is reported to undergo spontaneous remodeling with favorable clinical outcomes. DSM with arteriovenous shunt are aggressive lesions requiring prompt treatment [ 10, 25, 27]. Small cortical pial AVFs near the dural AVF can be associated. Like other arteriovenous shunt, congestive heart failure is a common manifestation in newborns due to cardiac overload. Cerebral venous hypertension and intracranial hemorrhage can also be seen in case the intracranial venous drainage is compromised by the thrombosed sinus/vein. MRI and MR angiography are usually obtained to better evaluate the brain parenchyma and for better evaluation of the angioarchitecture of the dural AVF [ 6, 12, 16] ( Fig. 6).
INTRACRANIAL VASCULAR MALFORMATIONS WITHOUT ARTERIOVENOUS SHUNTS
CM
CMs are low-flow capillary lesions made up of compact clusters of sponge-like vascular spaces without intervening neural parenchyma. They are also referred to as cavernous hemangiomas, cavernous angiomas, and cavernomas. Individuals who have received intracranial radiation are at a higher risk of developing CMs. Multiple CMs are common and linked to a familial predisposition [ 15]. While most CM show a benign course, symptomatic intracranial hemorrhage (ICH) can occur in pediatric age group and the estimated overall cumulative 5-year risk of symptomatic ICH is up to 20% [ 24]. In the absence of acute hemorrhage, CMs appear on noncontract CT as slightly hyperdense masses with little mass effect. After contrast enhancement, mild enhancement is observed. On MRI, the characteristic appearance of nonhemorrhagic CM is a sharply marginated, lobulated mass without surrounding edema. Different signal characteristics are usually observed in the lobules; for example, some areas show high signal intensity on T1- and T2-weighted images, others show low signal intensity on T2-weighted images, and still others show intermediate signal on T1 and variable signal on T2-weighted images ( Fig. 7). A DVA may be identified near the CM after contrast administration ( Fig. 8) [ 17, 24].
Developmental venous anomalies
DVA is a variant in venous development where either the cerebral cortex drains focally to the deep venous system, or the white matter drains to the cortical veins. A distinctive caput medusa sign is dilated medullary veins conversing on transcortical collecting vein. About 20% to 40% cases of DVAs are frequently observed in conjunction with CM ( Fig. 8). While DVAs are usually incidentally found and remain silent, the coexisting CM may cause hemorrhage [ 22].
Capillary telangiectasia
Capillary telangiectasias are small, dilated capillaries intermixed with normal brain parenchyma. They rarely bleed, and are most frequently located in the pons, and are usually discovered incidentally. Development of capillary telangiectasia has been reported after radiation [ 15]. While capillary telangiectasias in isolation are relatively benign they can occur in syndromes such as hereditary hemorrhagic telangiectasia. Most lesions are tiny and have sluggish flow, therefore traditional imaging modalities like CT and conventional T1- and T2-weighted MRI may not have adequately visualized them. Contrast enhanced T1-weighted images and GRE images are valuable in the diagnosis of capillary telangiectasia. Marked hypointensity is typically seen on GRE or SWI. After contrast enhancement, capillary telangiectasia shows moderate enhancement with faint, brush-like borders ( Fig. 9).
Sinus pericranii
Sinus pericranii is an abnormal connection between intracranial and extracranial vasculature presenting during childhood as a nontender palpable scalp lesion. Sinus pericranii can be congenital or secondary to trauma. Ultrasonography with color coded Doppler imaging and MRI can demonstrate abnormal communication between dural sinuses and superficial veins [ 18] ( Fig. 10). DSA is necessary to evaluate the flow dynamics, which determines timing and type of treatment if needed.
SUMMARY
All the cerebral vascular malformations seen in adults can be manifest in children as well, albeit with some differences as to morphology and presentation. Imaging plays a major role in the diagnosis, proper characterization, and evaluation of cerebral vascular malformations in children ( Table 1). Although DSA remains the gold standard in the diagnosis and characterization of these vascular malformations, advanced MRI and CT provide useful information relating to the association of vascular lesions to the surrounding intracranial structures as well as physiologic information, which cannot always be adequately evaluated with DSA. In unique cerebral vascular malformation requiring endovascular interventions, MRI and CT are essential in pretreatment planning as well as posttreatment evaluation for residual arteriovenous shunting in intracranial AVM or AVF.
Fig. 1.
arteriovenous malformation (aVM) with compact nidus. a : Non-contrast computed tomography (CT) image shows high density hematoma in the left occipital lobe (arrow). B : axial T2-weighted image shows compactly entangled flow voids mass (nidus, arrow) without any interposed normal brain tissue, which is surrounded by edematous parenchyma associated with hemorrhage. C : The nidus (arrow) is intensely enhanced on CT angiography. D : Vertebral arteriography reveals densely packed vascular nidus supplied by the left posterior cerebral artery, and early draining vein to the transverse sinus via superior cerebellar vein (arrows). 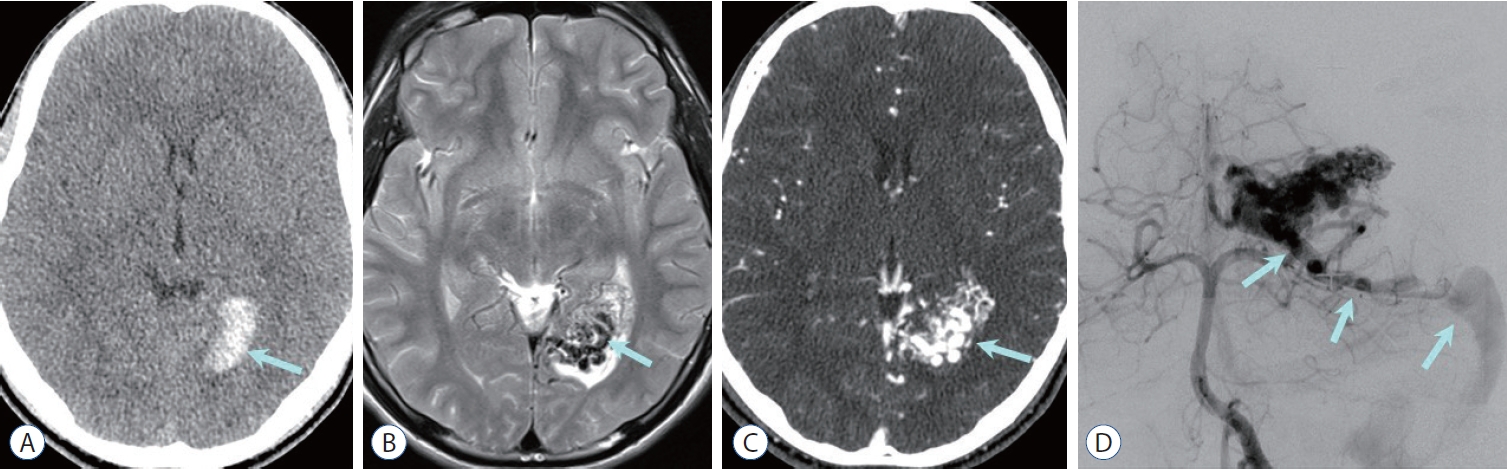
Fig. 2.
Cerebral proliferative angiopathy : diffuse or proliferative type arteriovenous malformation. a : axial T2-weighted image shows diffuse or proliferative type of nidus and interspersion of normal brain parenchyma involving right temporo-occipital lobes and basal ganglia. B : Contrast enhanced T1-weighted image shows diffuse network of enhancing vascular nidus and dilated draining veins (arrows). C : Susceptibility-weighted image shows multifocal blood products of dark signal intensity and dilated veins exhibiting high signal intensities reflecting rapid arteriovenous shunting (arrow). D : Magnetic resonance angiography reveals scattered nidus fed by multiple arteries (absence of a dominant feeder) including both anterior and posterior circulation, and transdural supplies from the external carotid artery (ECa) branches. E-I : anterior-posterior projection of angiographic images obtained after contrast injection at the right internal cerebral artery (E and F), left vertebral artery (G), and both ECas (H and I) reveal fuzzy appearance of nidus and scattered “puddling” of contrast which persists into the late arterial and venous phases. There are numerous feeders of normal size or moderately enlarged from internal cerebral artery, posterior cerebral artery, and ECa branches, and paucity of early venous drainage. 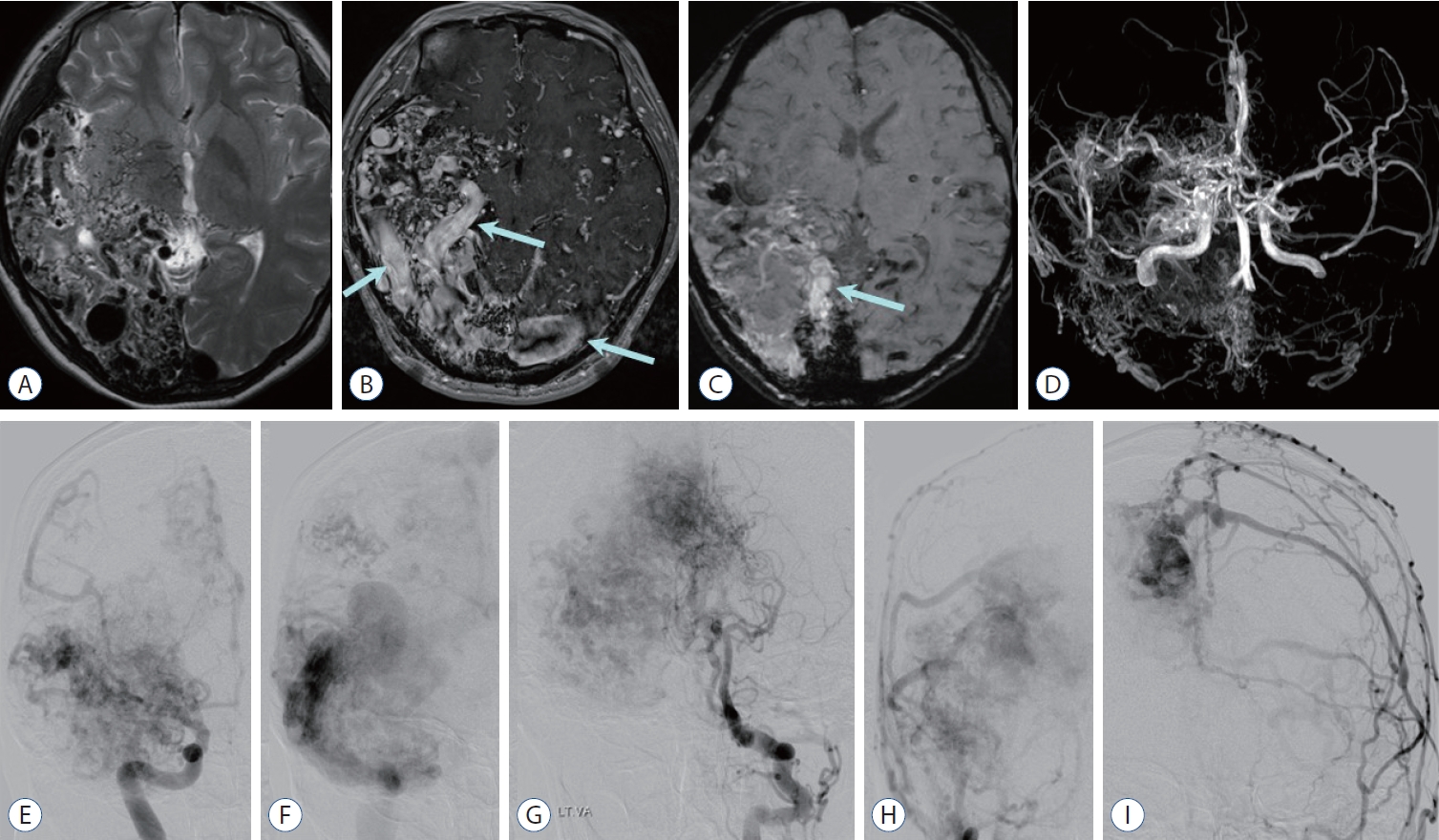
Fig. 3.
Choroidal type vein of Galen aneurysmal malformation (VGaM) in a neonate with congestive heart failure. a : Chest radiography shows enlarged heart in keeping with congestive heart failure. Coronal (B) and sagittal (C) cranial sonographic images show midline vascular mass (arrow) and distended ventricles. D : Color Doppler image demonstrates vein of Galen aneurysm with turbulent vascular flow. E : axial T2-weighted image shows large midline varix with adjacent multiple feeders (arrows), ventriculomegaly, and encephalomalacia in bilateral occipital lobes presumed from venous congestion and arterial steal from VGaM. F : Sagittal T1-weighted image shows prominent phase encoding pulsating artifact from the varix. Magnetic resonance angiography (G) reveals numerous small feeding arteries (arrows), which is more apparent on left vertebral artery angiography (H, arrows). 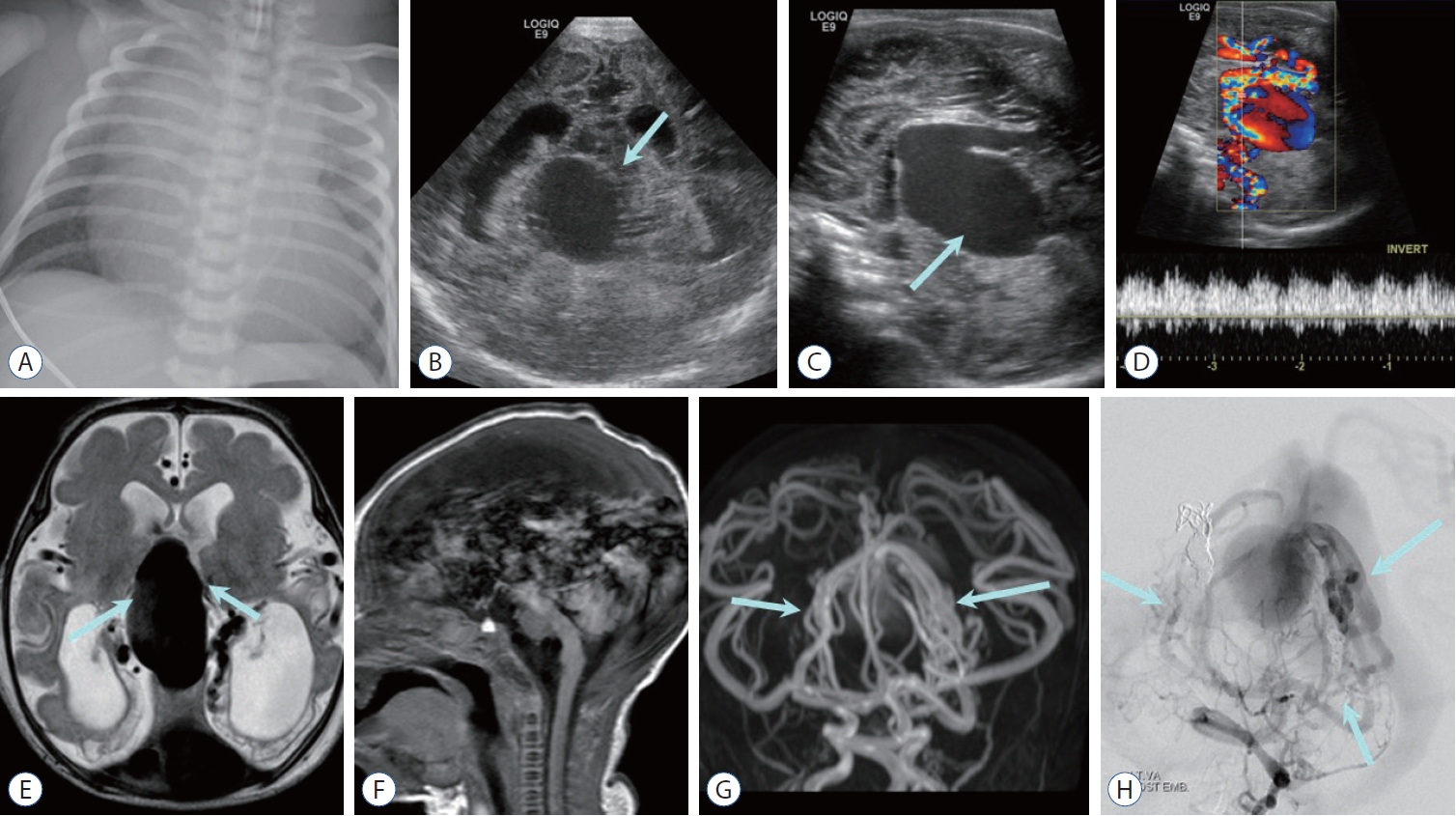
Fig. 4.
Mural type vein of Galen aneurysmal malformation in a 3-year-old boy. a : Noncontrast computed tomography image shows large central venous varix (arrows) and enlarged ventricles. B : T2-weighted image demonstrates dark signal intensity of the dilated vein (arrow) with phase misregistration artifact due to the vascular flow. C : Sagittal T1-weighted image demonstrates markedly dilated central vein (vein of Galen) connected to the falcine sinus (arrow) and prominent phase misregistration artifact. Note the hydrocephalus caused by mass effect of dilated central vein on cerebral aqueduct. D : Lateral projection of the left vertebral arteriography at the arterial phase shows connection (mural type) between the dilated left superior cerebellar artery (arrow) and dilated median prosencephalic vein of Markovski (vein of Galen). E : Lateral projection of the left vertebral arteriography after coil embolization reveals contrast filling the vein of Galen drained into the transverse sinus and jugular vein via the falcine sinus (arrow). Note the absence of the straight sinus. 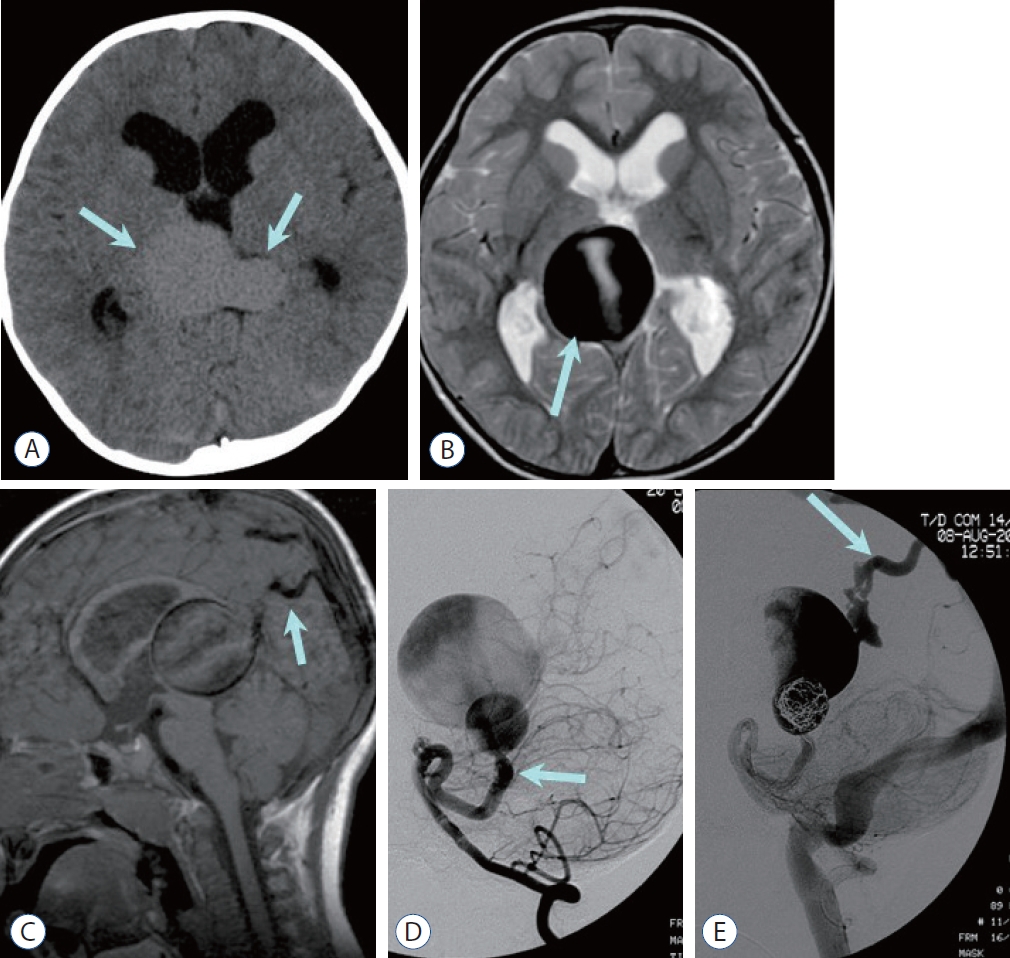
Fig. 5.
Pial arteriovenous fistula in a 2-year-old boy. Non-contrast computed tomography (CT) (a) shows high density venous pouch (V) on the surface of the left insula and a small round high density medially (arrow in a), which is traced as a signal void tubular structure (arrow in B) on T2-weighted image suggesting dilated high-flow feeder. The dilated venous pouch is also seen as dark signal intensity on T2-weighted image (B). an axial image (C) and a 3D-reconstruction image (D) of CT angiography demonstrate dilatated left distal middle cerebral artery (MCa) as a feeder artery (arrow, C and D) with intense enhancement (C), while the venous pouch is not enhanced suggesting thrombotic occlusion (V). E : anterior-posterior projection of the left internal cerebral angiography shows dilated MCa feeder (arrow) and non-visualized venous pouch. 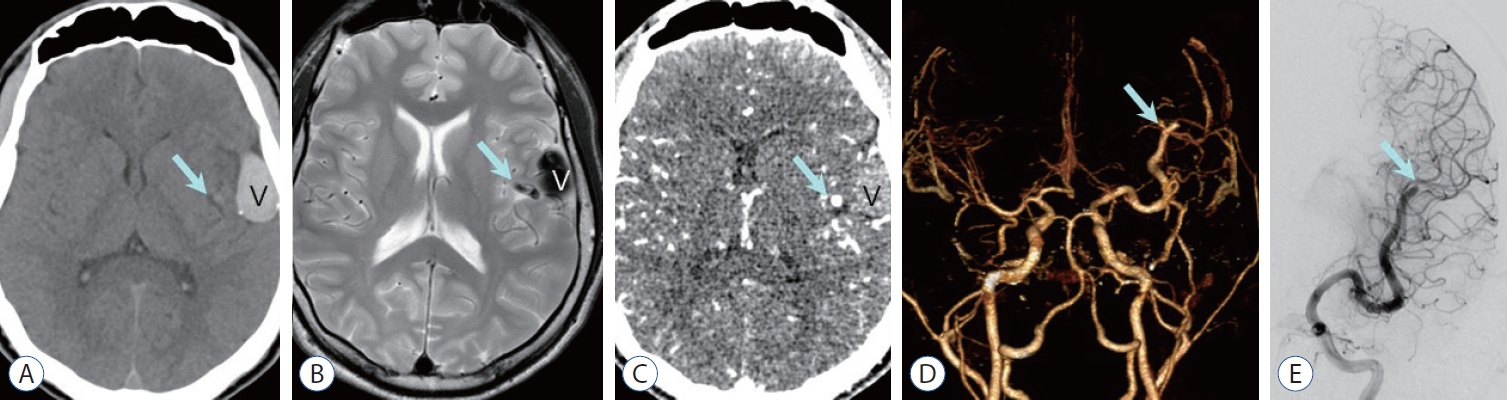
Fig. 6.
Dural arteriovenous fistula with dural sinus malformation in a 7-month-old boy. a massively dilated torcular herophilli (arrow) is visualized on axial T2-weighted image (a) and sagittal contrast enhanced T1-weighted image (B). C : Contrast enhanced magnetic resonance venography shows tortuous vertebral artery branches (arrow) supplying dilated torcular herophilli and transverse sinus. D : Lateral projection of left vertebral arteriography shows engorged posterior meningeal branches (arrow) communicating to the torcula herophilli with reflux to superior sagittal sinus. 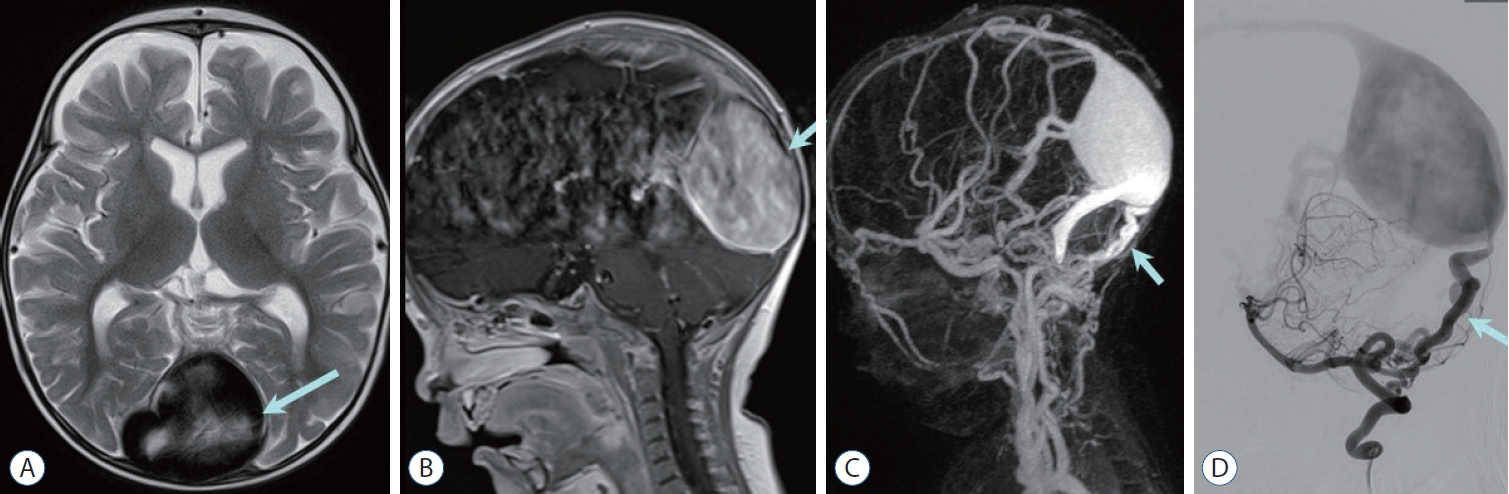
Fig. 7.
Cavernous malformation. a : Non-contrast computed tomography shows high-attenuated mass containing multiple calcifications in the left frontal lobe (arrow). B : Left frontal mass is seen as central heterogeneous high signal intensity surrounded by dark area of hemosiderin deposition, so called popcorn-like appearance (arrow) on axial T2-weighted image. C : Central portion of the mass (arrow) shows heterogeneous enhancement on contrast enhanced T1-weighted image. D : Prominent blooming dark signal intensity (arrow) is seen on gradient echo image suggesting blood product. 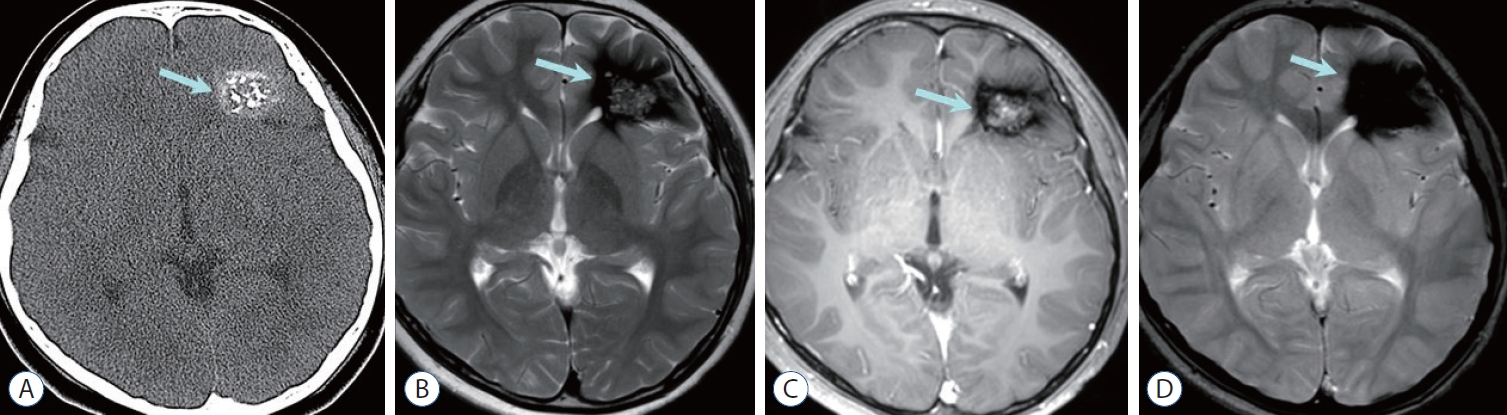
Fig. 8.
Developmental venous anomaly (DVa). a and B : axial T2-weighted images show curvilinear collecting vein suggesting DVa (arrow in a) and adjacent small hemorrhagic cysts surrounded by edema (arrow in B) suggesting coexisting cavernous malformation. C : Contrast enhanced T1-weighted image reveals enhancing collecting vein and dilated medullary veins showing ‘caput medusa’ sign (arrow). D : Dilated medullary veins converging on transcortical collecting vein (arrows) are well demonstrated on a delayed phase of the vertebral arteriography. 
Fig. 9.
Capillary telangiectasia. a : axial T1-weighted image shows a small, poorly marginated, pontine lesion (arrow) of low signal intensity without mass effect nor edema (arrow). B : The pontine lesion (arrow) is very subtle and faintly visualized on T2-weighted image. Contrast enhanced axial (C) and sagittal (D) T1-weighted images reveal stippled/brush-like enhancement (arrow). E : Profound hypointense pontine lesion (arrow) is well visualized on susceptibility-weighted image. 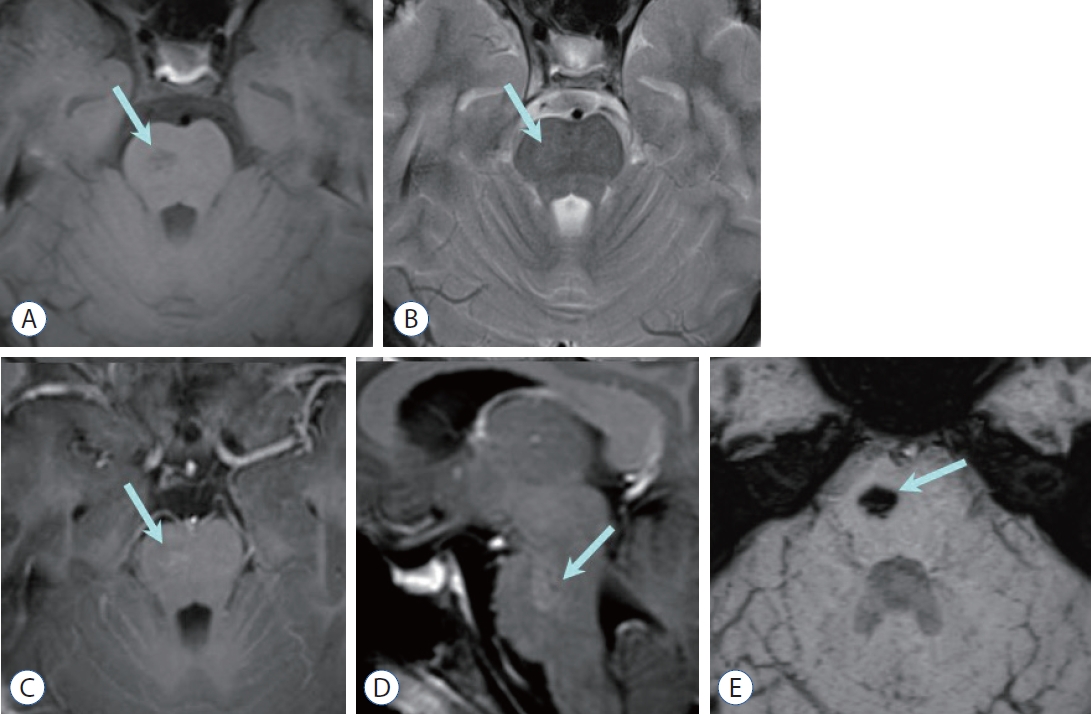
Fig. 10.
Sinus pericranii. a : Sonography shows lobulated cystic lesions in the scalp at the vertex, with a connection to the underlying dural sinus (arrow). Color Doppler image (B) demonstrates prominent vascular flow (arrow in B) connecting the scalp lesion and dural sinus, with pulsatile spectral waves (C). Coronal T2-weighted image (D) and contrast enhanced T1-weighted image (E) show signal void (arrow in D) or enhancement (arrow in E) of the scalp vein connecting to the superior sagittal sinus. 
Table 1.
Summary of characteristics of intracranial vascular malformations
|
Common location |
Hemorrhage risk |
Best imaging clues |
|
Intracranial vascular malformations with arteriovenous shunt |
|
|
|
|
AVM |
|
Very high (4-5% per year, cumulative) |
‘Flow voids’ on MRI |
|
Vein of Galen aneurysmal malformation |
Prosencephalic vein |
Low (but hydrocephalus, brain damage common) |
Large midline varix in neonate |
|
Dural AVF |
Transverse or sigmoid sinus |
Varies with venous drainage |
Enlarged meningeal arteries with network of tiny vessels in wall of thrombosed dural venous sinus |
|
Intracranial vascular malformations without arteriovenous shunt |
|
|
|
|
Cavernous malformation |
2/3 solitary (sporadic); 1/3 multiple (familial) |
High (cumulative 5-year risk of symptomatic ICH : 20%) |
‘Popcorn ball’ |
|
Multifocal black dots in familial cases on T2*/SWI |
|
Developmental venous anomaly |
Deep white matter usually near ventricle |
Extremely low unless coexisting cavernous malformation |
Caput medusa sign |
|
Sinus pericranii |
Midline or paramedian scalp |
Extremely low unless direct trauma |
Vascular scalp mass connecting through skull defect to intracranial venous circulation |
|
Capillary telangiectasia |
Pons |
Extremely low unless coexisting cavernous malformation |
Faint brush-like enhancement, hypointense on T2*/ SWI |
References
1. Alvarez H, Garcia Monaco R, Rodesch G, Sachet M, Krings T, Lasjaunias P : Vein of galen aneurysmal malformations. Neuroimaging Clin N Am 17 : 189-206, 2007
2. Burch EA, Orbach DB : Pediatric central nervous system vascular malformations. Pediatr Radiol 45 Suppl 3 : S463-S472, 2015
3. Chen CJ, Ding D, Derdeyn CP, Lanzino G, Friedlander RM, Southerland AM, et al : Brain arteriovenous malformations: a review of natural history, pathobiology, and interventions. Neurology 95 : 917-927, 2020
4. Darsaut TE, Guzman R, Marcellus ML, Edwards MS, Tian L, Do HM, et al : Management of pediatric intracranial arteriovenous malformations: experience with multimodality therapy. Neurosurgery 69 : 540-556; discussion 556, 2011
5. Geibprasert S, Pongpech S, Jiarakongmun P, Shroff MM, Armstrong DC, Krings T : Radiologic assessment of brain arteriovenous malformations: what clinicians need to know. Radiographics 30 : 483-501, 2010
6. Hacein-Bey L, Konstas AA, Pile-Spellman J : Natural history, current concepts, classification, factors impacting endovascular therapy, and pathophysiology of cerebral and spinal dural arteriovenous fistulas. Clin Neurol Neurosurg 121 : 64-75, 2014
7. Hetts SW, Moftakhar P, Maluste N, Fullerton HJ, Cooke DL, Amans MR, et al : Pediatric intracranial dural arteriovenous fistulas: age-related differences in clinical features, angioarchitecture, and treatment outcomes. J Neurosurg Pediatr 18 : 602-610, 2016
8. Jaimes C, Machado-Rivas F, Chen K, Bedoya MA, Yang E, Orbach DB : Brain injury in fetuses with vein of Galen malformation and nongalenic arteriovenous fistulas: static snapshot or a portent of more? AJNR Am J Neuroradiol 43 : 1036-1041, 2022
9. Krings T, Geibprasert S, Terbrugge K : Classification and endovascular management of pediatric cerebral vascular malformations. Neurosurg Clin N Am 21 : 463-482, 2010
10. Liby P, Lomachinsky V, Petrak B, Kyncl M, Charvat F, Padr R, et al : Torcular dural sinus malformations: a single-center case series and a review of literature. Childs Nerv Syst 36 : 333-341, 2020
11. LoPresti MA, Ravindra VM, Pyarali M, Goethe E, Gadgil N, Wagner K, et al : Pediatric intracranial arteriovenous malformations: a single-center experience. J Neurosurg Pediatr 25 : 151-158, 2019
12. Maleknia PD, Hale AT, Savage C, Blount JP, Rocque BG, Rozzelle CJ, et al : Characteristics and outcomes of pediatric dural arteriovenous fistulas: a systematic review. Childs Nerv Syst 40 : 197-204, 2024
13. Mansmann U, Meisel J, Brock M, Rodesch G, Alvarez H, Lasjaunias P : Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery 46 : 272-279; discussion 279-281, 2000
14. Mokin M, Dumont TM, Levy EI : Novel multimodality imaging techniques for diagnosis and evaluation of arteriovenous malformations. Neurol Clin 32 : 225-236, 2014
15. Montaser A, Smith ER : Intracranial vascular abnormalities in children. Pediatr Clin North Am 68 : 825-843, 2021
16. Mossa-Basha M, Chen J, Gandhi D : Imaging of cerebral arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am 23 : 27-42, 2012
17. Paddock M, Lanham S, Gill K, Sinha S, Connolly DJA : Pediatric cerebral cavernous malformations. Pediatr Neurol 116 : 74-83, 2021
18. Rai Y, Ogiwara H : Atretic cephalocele associated with sinus pericranii: a single-center analysis. Childs Nerv Syst 40 : 543-547, 2024
19. Ravindra VM, Bollo RJ, Eli IM, Griauzde J, Lanpher A, Klein J, et al : A study of pediatric cerebral arteriovenous malformations: clinical presentation, radiological features, and long-term functional and educational outcomes with predictors of sustained neurological deficits. J Neurosurg Pediatr 24 : 1-8, 2019
20. Recinos PF, Rahmathulla G, Pearl M, Recinos VR, Jallo GI, Gailloud P, et al : Vein of Galen malformations: epidemiology, clinical presentations, management. Neurosurg Clin N Am 23 : 165-177, 2012
21. Requejo F, Teplisky D, Dutra MLG, Mouratian DM, Kikano R, Nguyen TN, et al : Pediatric interventional neuroradiology. Semin Neurol 43 : 408-418, 2023
22. Ruíz DS, Yilmaz H, Gailloud P : Cerebral developmental venous anomalies: current concepts. Ann Neurol 66 : 271-283, 2009
23. Sabayan B, Lineback C, Viswanathan A, Leslie-Mazwi TM, Shaibani A : Central nervous system vascular malformations: a clinical review. Ann Clin Transl Neurol 8 : 504-522, 2021
24. Santos AN, Rauschenbach L, Saban D, Chen B, Herten A, Dinger TF, et al : Natural course of cerebral cavernous malformations in children: a five-year follow-up study. Stroke 53 : 817-824, 2022
25. Sarma A, Martin D, Pruthi S, Jones R, Little SB : Imaging the cerebral veins in pediatric patients: beyond dural venous sinus thrombosis. Radiographics 43 : e2201292023
26. Shtaya A, Millar J, Sparrow O : Multimodality management and outcomes of brain arterio-venous malformations (AVMs) in children: personal experience and review of the literature, with specific emphasis on age at first AVM bleed. Childs Nerv Syst 33 : 573-581, 2017
27. Zuniega RRA, Santos JA, Galsim RJG, Elevazo JS : Neonatal giant dural sinus ectasia: a multimodality imaging approach. BMJ Case Rep 14 : e2424392021
|
|


























