Infection of Cranioplasty Seen Twenty Years Later
Article information
Abstract
Cranioplasty is performed using autograft and allograft materials on patients to whom craniectomy was applied previously due to the facts that, this region is open to trauma and the scalp makes irritation and pressure onto the brain paranchyma causing brain atrophy and convulsions. Dramatical improvement of neurological deficits, control of convulsions and partial prevention of cerebral atrophy are achieved after these operations. One of the most important complications of cranioplasty is late infection. Here, we report a 43-year-old male patient admitted with the history of purulant discharge from the right temporal incission site for one year to whom cranioplasty had been performed with allograft material 20 days after craniectomy which had been performed in 1989. Allograft cranioplasty material was removed and cranioplasty was performed using new allograft material with the diagnosis of late cranioplasty infection.
INTRODUCTION
Cranioplasty is performed for calvarial defects due to the facts that this region is vulnerable to trauma, calvarial defects may cause cerebral atrophy and convulsions or for cosmetic purposes7,10,12). Improvement of neurological deficits, control of convulsions and partial prevention of cerebral atrophy are achieved after these operations12,14).
Edwards and Ousterhout4) advocated autogenous bone graft being the most appropriate cranioplasty material for children and adolescents however allogreft material was emphasized as the ideal graft for adults3,5).
One of the most important complications of cranioplasty is late infection or foreign body reaction mimicking infection2,3,7,14). Infections are usually seen 3-10 months after the cranioplasty operations3,7) however late infections presenting 20 years after cranioplasty operations as seen in our case are very rare.
CASE REPORT
A 43-year-old male patient was admitted to our hospital with the complaint of purulant discharge from the right temporal inscission site for one year. Right temporal craniectomy had been performed for depressed skull fracture in 1989. Twenty days after craniectomy, cranioplasty had been performed to craniectomized region with allograft material.
Systemic examination was unremarkable. Laboratory examination revealed normal levels of erythrocyte sedimentation rate (5 mm/h), C-reactive protein (0.2 mg/L) and white blood cells (7.9×109/L). There was no microbial yield in culture of purulant discharge.
In cranial computed tomography (CT) scan, allograft cranioplasty material and calcified tissue together was seen as hyperdense area (Fig. 1A). In cranial MRI scan, allograft cranioplasty material together with calcified and fibrotic tissue thereunder was seen (Fig. 1B).
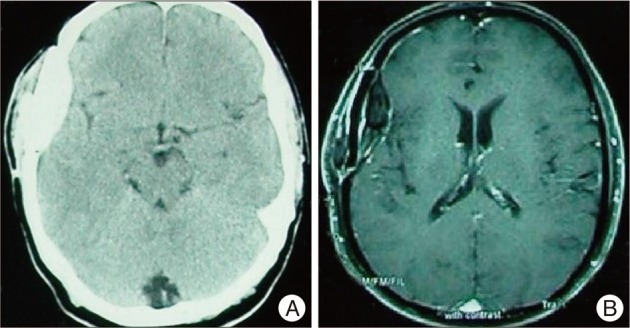
A : Cranial computed tomography scan shows allograft cranioplasty material and calcified tissue together seen as hyperdense area. B : Cranial magnetic resonance imaging scan shows allograft cranioplasty material together with calcified and fibrotic tissue thereunder.
During the operation; white-yellow coloured tissue layer was seen on the surface of cranioplasty material (Fig. 2A). After removal of the cranioplasty material; calcified, fibrotic and white-yellow coloured tissue layer 5 mm in thickness was seen in epidural area (Fig. 2B). There was no physical change in cranioplasty material after removal of surrounding tissues (Fig. 2C).

A : On the surface of the cranioplasty material, white-yellow coloured layer is seen. B : After removal of cranioplasty material; white-yellow coloured calcified and fibrotic tissue is seen in epidural area. C : After removal of surrounding tissues, cranioplasty material is seen with no physical change.
No microbial yield was detected in culture of cranioplasty material and surrounding tissues. The postoperative course was entirely uneventful.
DISCUSSION
Cranioplasy is performed to cranial defects for functional and cosmetic purposes5,7,12,14). In craniectomized patients; scalp herniates towards brain through defect and makes irritation to brain by means of atmospheric pressure leading to cerebral atrophy and convulsions. Improvement of neurological deficits, control of convulsions and partial prevention of cerebral atrophy are achieved after cranioplasty in these patients12,14). In our case there was no neurological deficit and history of convulsion.
One of the most important complications of cranioplasty is late infection2,14). Post-cranioplasty infections are seen 3-10 months after cranioplasty1,3,7). However late infections seen 20 years after cranioplasty are very rare8). Our case presented with purulant discharge 20 years after the cranioplasty.
Infection rates after cranioplasty are 1-13.5%3,5,7,14). Infection rates are higher in patients previously operated for cranioplasty or another cranial operation and whose previous operations include frontal sinus3,7,13,14). Infective complications are higher when time passing between craniectomy and cranioplasty is not long enough6,9,11,13-15). In our case, the time interval between craniectomy and cranioplasty is only 20 days which is not long enough and thought to be facilitating infection.
No statistical significance was found between the infection rates and size of cranioplasty material used, choice of allograft or autograft, the prophylactical antibiotic used, age, sex, site and duration of operation14).
Edwards and Ousterhout4) advocated that autograft is the ideal cranioplasty material for children and adolescents while allograft material was emphasized to be more appropriate for adults and previously infected patients3,5,7). The previously used cranioplasty material in our case was allograft and we replaced it with allograft material again.
Cheng et al.3) emphasized that neither negative culture of infected material rules out infection nor positive culture certainly indicates it. The culture of purulant discharge and removed graft with surrounding reactive tissues were negative in our case but we still diagnosed the case as late cranioplasty infection.
Detection of air bubbles and dural contrast enhancement in cranial CT are not signs of infection due to the fact that these findings are detected in infected as well as uninfected patients (1). For this reason, the most reliable indicator of infection is the clinical presentation of the patient as always instead of imaging techniques and laboratory findings only.
CONCLUSION
In our case, infection of cranioplasty presented 20 years after the operation which is very rare in the literature. However, possibility of this late complication should be appreciated and that follow-up period after cranioplasty operations should not be short considering late cranioplasty infections.