Successful Treatment of Tracheoinnominate Artery Fistula Following Tracheostomy in a Patient with Cerebrovascular Disease
Article information
Abstract
Tracheoinnominate artery fistula is a critical complication of tracheostomy. The most important factors influencing patient outcome are prompt diagnosis, immediate control of bleeding with a patent airway, and emergency operation with or without interruption of the innominate artery. Here, we report a case of tracheoinnominate artery fistula in a 40-year-old woman with cerebrovascular accident who was successfully managed with an aorta-axillary artery bypass.
INTRODUCTION
Tracheostomy is the most effective and popular procedure for patients suffering from pulmonary problems or those requiring long-term ventilatory support in the neurosurgical field. However, there can be some complications. Tracheoinnominate artery fistula (TIAF) is a rare complication of tracheostomy that occurs in <1% of patients with a tracheostomy cuff6,9). As this disease often presents with sentinel bleed followed by massive hemorrhage, it is life-threatening if not promptly diagnosed and treated.
Here, we describe a case of TIAF that was successfully managed with aorta-axillary artery graft bypass for preserving blood flow.
CASE REPORT
A 46-year-old woman visited the emergency room with left hemiparesis and dysarthria. She was diagnosed with an intracerebral hemorrhage (ICH) at the right basal ganglia by brain computed tomography (CT) (Fig. 1A). Three days later, a chest X-ray revealed a diffuse haziness in both lung fields (Fig. 1B). Her pulmonary status was further complicated by an inability to cooperate with respiratory toilet and total opacification of both lungs on the chest X-ray. She was intubated due to aspiration pneumonia. Her condition was worsened despite intravenous administration of culture-specific antibiotics and repeated sputum drainage. A tracheostomy was performed two weeks later, and the patient was ventilated via a low pressure tube inserted through the second tracheal ring. Thereafter, she stabilized and began to improve.
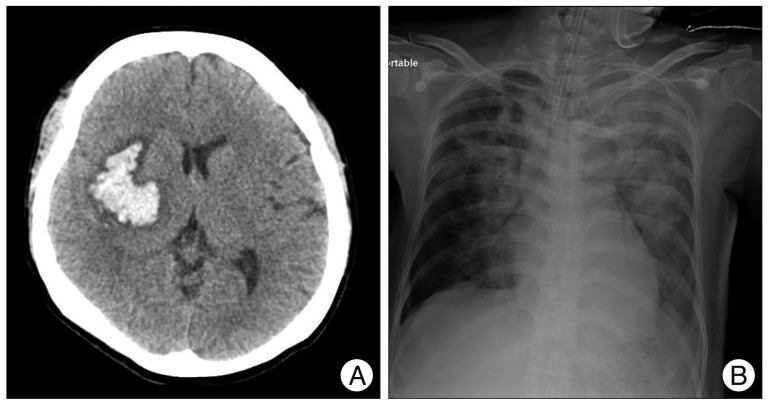
A 46-year-old woman visited the emergency room with left hemiparesis and dysarthria. Brain computed tomography demonstrated an intracerebral hemorrhage at the right basal ganglia (A). Three days later, a chest X-ray revealed diffuse haziness in both lung fields induced by aspiration pneumonia (B).
Three months after the tracheal intubation, massive bleeding was detected from the tracheal stoma on tracheal suction and the patient vital signs became unstable. Her blood pressure was below 80/60 and with 40% oxygen saturation. The tracheostomy tube was replaced with a flexible endotracheal tube and the endotracheal tube cuff was inflated until adequate ventilation and bleeding control was achieved. Portable fiber optic bronchoscopy could not identify the source of the hemorrhage at the anterior wall of the midtrachea. A thoracic CT scan demonstrated that the tip of the endotracheal tube was in contact with the tracheal wall in the back of a tracheoinnominate artery (Fig. 2). Suspecting TIAF, we brought the patient to the interventional radiology suite. Emergent aortic arch angiography was performed through the right common femoral artery. Catheterization of the aortic arch did not locate the fistula, but upon selective catheterization of the innominate artery, the fistula was clearly visible near the origin of the common carotid artery (Fig. 3). Embolization of the innominate artery using coils was not recommended by the interventionalist because of an occlusion of the right common carotid artery. We also were worry of unexpected results, such as ischemia caused by occlusion of the common carotid artery.
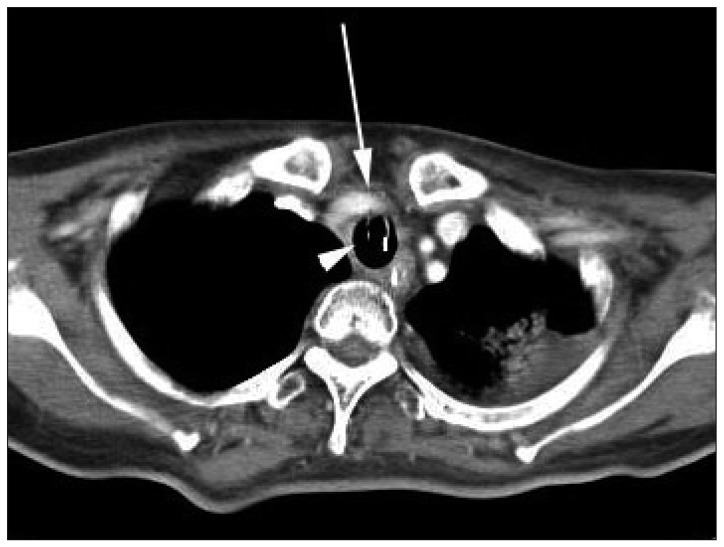
Contrast enhanced thoracic computed tomography demonstrated that an overinflated endotracheal tube cuff (white arrowhead) was controlling bleeding from fistula site by compressing the innominate artery (white arrow).
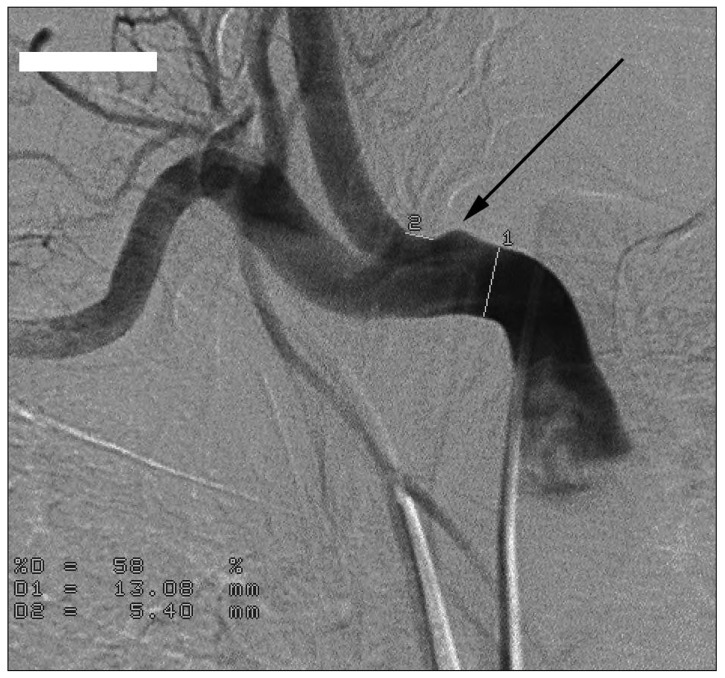
An angiogram of the innominate artery demonstrated outpouching near the right common carotid artery (black arrow). For this reason, we selected a surgical management instead of an endovascular option.
The operative procedure to control the fistula and maintain adequate blood flow to the brain consisted of first performing a right aorta-axillary artery bypass using a synthetic graft, followed by resection of the 3 mm fistula and oversewing the divided ends of the innominate artery. The defect in the trachea was approximated with 3-0 Vicryl sutures, and autologous tissue was interposed. Control of the fistula was confirmed by thoracic CT angiography with three-dimensional (3D) reconstruction (Fig. 4A).
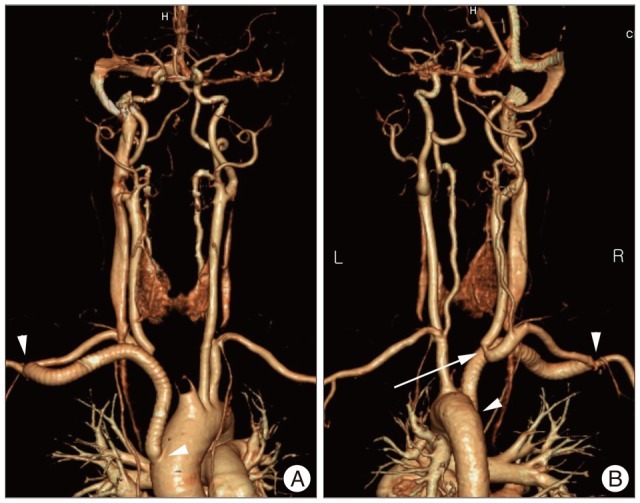
Anterior (A) and posterior (B) views of thoracic computed tomographic angiography with three-dimensional reconstruction show good perfusion of the carotid artery, subclavian artery, and intracranial circulation after aorta-axillary artery bypass using a synthetic graft. Aorta-axillary artery graft bypass is visible between two white arrow heads and there is narrow at the junction between the innominate artery and the common carotid artery (white arrow) owing to an adjacent fistula site.
Postoperatively, the patient exhibited no evidence of graft infection or erosion and recurrent bleeding. The patient's focal neurologic deficits remained unchanged. She was discharged after three months and continued to do well.
DISCUSSION
TIAF is an uncommon and life-threatening complication of tracheostomy with a reported incidence of 0.7%6,9). However, the mortality rate is nearly 100% if it is not treated urgently with definitive management. TIAF occurs most frequently within the first three weeks after tracheostomy in 72% of patients, but has been reported to occur many months postoperatively2,5,7). The initial bleeding may be mild, but these sentinel bleeding events often precede a massive hemorrhage. In addition, bleeding from TIAF can occur without a recognized sentinel bleed6). In order to control these massive hemorrhages emergently, several methods for achieving temporary hemostasis can be performed. The best temporary maneuver is to overinflate the tracheostomy tube cuff7,11). If the hemorrhage does not stop despite overinflating the cuff, direct digital compression can be applied to the anterior tracheal wall through the stoma to compress the vessel wall11).
The cause of TIAF is pressure necrosis of the anterior tracheal wall resulting from the tracheostomy cuff or tip, which causes erosion of the trachea and innominate artery10). In addition, several factors are known to promote the formation of this fatal complication, including low tracheostomy tube placement, overinflation of the tracheostomy cuff, a malpositioned tracheostomy tube tip, movement transmitted from the ventilator, local infection of a tracheostomy wound, and anatomical variations of an abnormally horizontal or high innominate artery3,12). Early diagnosis is the most important factor for successful management of a TIAF. Bronchoscopy, arteriography, and CT angiography with 3D reconstruction can be helpful in diagnosing TIAF, but these studies often fail to confirm the diagnosis due to their low sensitivity (20% to 30%)8). During the acute bleeding period, bronchoscopic examination is very difficult as the entire tracheal tree is filled with blood12). However, of the various angiographic techniques, an oblique subtraction view of a selective innominate arterial injection had the highest diagnostic value, although there are not enough data to assess the sensitivity and specificity of this diagnostic method12).
Our patient was intubated as aspiration pneumonia occurred three days after she presented with an ICH. We decided to perform tracheostomy two weeks later because her pneumonia had not improved. However, three months after tracheostomy, active bleeding was noted at the stoma site. We exchanged the tracheostomy tube with an endotracheal tube and the bleeding was controlled as soon as the balloon was inflated. We suspected TIAF which is a very rare complication of tracheostomy. Therefore, we assumed the active bleeding was from the granulation tissue instead of TIAF. We performed a bronchoscopy and thoracic CT scan, both of which failed to reveal pathologic conditions. To inspect for TIAF, aortic arch arteriography was performed via right femoral access by an interventionalist. Selective innominate angiography revealed outpouching at the innominate artery near the right common carotid artery (Fig. 3). We failed to achieve an early diagnosis. Several factors were contributed to the diagnosis delay, such as an active stomal bleeding without sentinel bleed and the onset at 3 months.
TIAF requires emergent and definitive surgical management. Generally, two basic management techniques have been introduced. One is maintaining flow through either direct repair of the defect or by interposition grafting, and the other is interrupting flow by simple ligation or resection of the innominate artery, while attempting to preserve the right carotid-right subclavian junction4). Most authors advocate the interruption of flow because reconstruction of the innominate artery for maintaining the flow has a high risk of rebleeding (60-80%)4,5,7,12). Therefore, ligation of the innominate artery is the treatment of choice due to decrease the rebleeding rate and mortality. Also, there is a lack of reports suggesting significant neurologic sequelae or vascular complications associated with ligation of the innominate artery. In fact, the risk of stroke is quite low following innominate artery ligation because the subclavian artery is filled with significant retrograde flow from the right vertebral artery. However, several reports indicated a significant risk of neurologic sequelae or vascular complications in approximately 10% of patients after innominate artery ligation1,3,5). If there are severe atherosclerotic changes in the right common carotid artery, right vertebral artery, and/or left common carotid artery stenosis/occlusion, ligation of the innominate artery should be considered significantly.
In our case, patient presented with left hemiparesis induced by ICH. If the patient aggravates left hemiparesis induced right ischemia due to the lack of blood flow of the right common carotid artery after innominate artery ligation, it is a critical and unfavorable condition. To make matters worse, right arm weakness may also develop due to the lack of blood flow in the right axillary artery. For this reason, we decided to perform an aorta-axillary artery bypass to ensure antegrade flow, followed by resection of the innominate artery. Postoperative thoracic CT angiography demonstrated that there was narrow at the junction between the innominate artery and the common carotid artery owing to an adjacent fistula site (Fig. 4B). It can be increased a risk of thromboembolic events because of occurring the turbulent flow of blood after poststenotic dilatation. We consider that it is necessary to use additional antiplatelet agents. Graft failure and rebleeding have not been reported for situations in which vascular continuity and antegrade flow are reestablished using clean inflow and outflow targets. Although reports have commented on the relative safety of innominate artery ligation, an aorta-axillary artery bypass is an easy method for restoring vascular continuity while protecting against the risk of rebleeding4).
CONCLUSION
TIAF is the most critical and life-threatening complication of tracheostomy in neurosurgical patients. The most important factors affecting outcome are prompt diagnosis, immediate control of bleeding with a patent airway, and emergency operation with or without interruption of the innominate artery. Although many reports recommend interruption of the innominate artery, an aorta-axillary artery bypass graft should be considered a useful treatment for TIAF in patients with severe stenosis/occlusion of the left common carotid artery, severe atherosclerosis, and brain ischemic or hemorrhagic insults.