Intracerebral Hemorrhage Following Evacuation of a Chronic Subdural Hematoma
Article information
Abstract
Burr hole drainage has been widely used to treat chronic subdural hematomas (SDH), and most of them are easily treated by simple trephination and drainage. However, various complications, such as, hematoma recurrence, infection, seizure, cerebral edema, tension pneumocephalus and failure of the brain to expand due to cerebro-cranial disproportion may develop after chronic SDH drainage. Among them, intracerebral hemorrhage after evacuation of a recurrent chronic SDH is very rare. Here, we report a fatal case of delayed intracerebral hemorrhage caused by coagulopathy following evacuation of a chronic SDH. Possible pathogenic mechanisms of this unfavorable complication are discussed and a review of pertinent literature is included.
INTRODUCTION
Chronic subdural hematoma (SDH) is known to have a good prognosis after simple burr hole drainage, and thus, most neurosurgeons do not consider chronic SDH seriously. However, unexpected neurological deterioration may occasionally occur after chronic SDH drainage. Contralateral or bilateral development of an acute subdural hematoma immediately after removal of chronic SDH have been previously reported to be rare but devastating postoperative complications3,12). However, massive intracerebral hemorrhage (ICH) caused by coagulopathy after evacuation of a recurrent chronic SDH has not been previously reported. Here, we report a rare case of fulminant ICH after the evacuation of a chronic SDH and include a review of the literature.
CASE REPORT
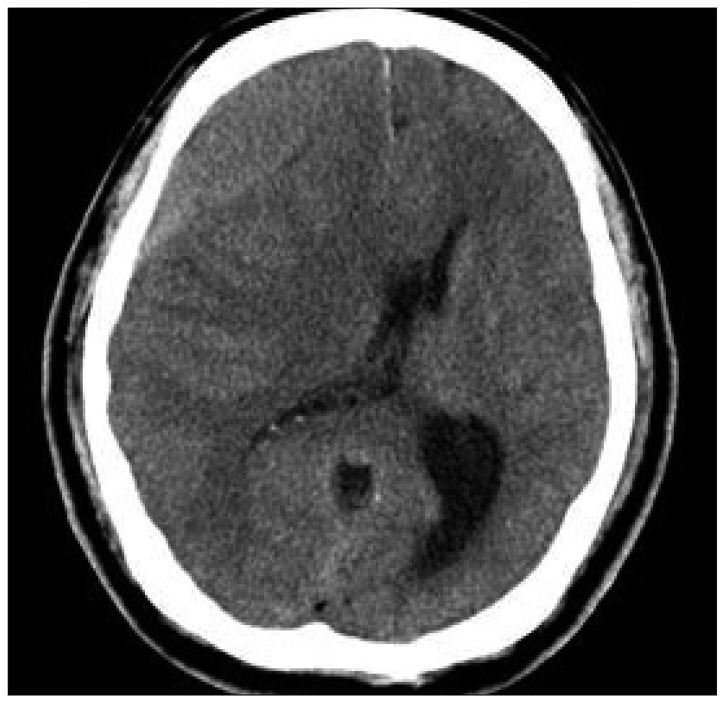
A 62-year-old man was admitted to our institute with a 3-week history of trivial head injury with complaints of mild headache and left side motor weakness. Neurologic examination revealed left hemiparesis (Grade V/Grade IV). He was afebrile and results of blood tests, including erythrocyte sedimentation rate and C-reactive protein, were all within normal limits. He had taken low dose aspirin 100 mg per day for 4 years, but routine laboratory test results, which included platelet count, prothrombin time (PT), activated partial thromboplastin time (aPTT) and bleeding time were within normal limits. Brain computed tomography (CT) scan depicted a hypodense lesion in the right frontotemporal (F-T) region, suggesting chronic SDH with midline shift (Fig. 1). The hematoma was evacuated through one burr hole using a 5-L catheter under local anesthesia; dark old blood was removed and no evidence of active bleeding was confirmed during the operation. After setting a simple closed system in the subdural space, the wound was closed layer by layer and the operation was completed in the usual manner. On the postoperative CT scan performed at the next day, we could find the resolution of hematoma without midline shifting (Fig. 2). Postoperatively, the headache was immediately relieved and the patient was able to ambulate independently without difficulty, after 5-L catheter removal. Hematological investigation revealed prolongation of PT prolongation to 14.0 sec but other parameters including fibrinogen were all normal range after trephination surgery. However, 3 days after the operation, the patient's level of consciousness gradually deteriorated to a drowsy state, and brain CT revealed recurrent SDH on Rt. F-T area (Fig. 3). The hematoma was re-evacuated through one burr hole using a 5-L catheter under local anesthesia; dark old blood was removed and no evidence of active bleeding was also confirmed. After setting a simple closed system in the subdural space, the wound was closed layer by layer and the operation was completed without any problem. After revision surgery, his mental status was recovered to alert and follow-up CT scan at following day after revision surgery revealed the resolution of hematoma without midline shifting (Fig. 4). However, hematological investigation revealed prolongation of PT to 14.1 sec and a decline of fibrinogen to 81 mg/dL (normal range >150 mg/dL). As a result, cryoprecipitate, fresh frozen plasma, and platelet concentrates were transfused. However, 5 days after revision surgery, the patient's level of consciousness deteriorated to semicomatose state. Brain CT revealed inracerebral hemorrhage in right basal ganglia and with midline shifting (Fig. 5). At this time, despite continuous replacement of coagulation factors, hematological investigation revealed disseminated intravascular coagulation (DIC); PT prolongation (14.5 sec), decreased fibrinogen (27 mg/dL), elevated fibrin degradation product test (10.5 Ml/mL) and D-dimer (575 mg/dL). Emergency decompressive surgery was planned, but his family refused the operation for profound neurological deficits and hematological laboratory results of DIC. Despite conservative treatment to lower intracranial pressure and normalize the hematological parameters, his condition deteriorated rapidly, culminating in multiple organ failure and cardiorespiratory arrest with dilated non-reactive pupils. He died on the thirteenth postoperative day after the initial operation.
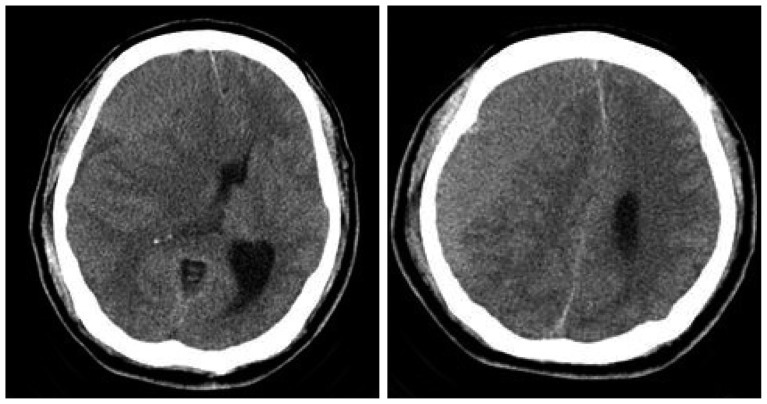
Preoperative computed tomography scan reveals isodense right chronic subdural hematoma with midline shifting.
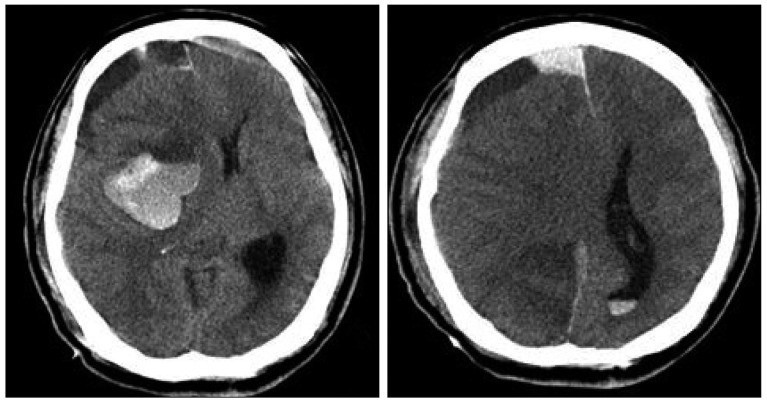
Postoperative computed tomography scan performed at the next day after trephination shows resolution of hematoma without midline shifting.
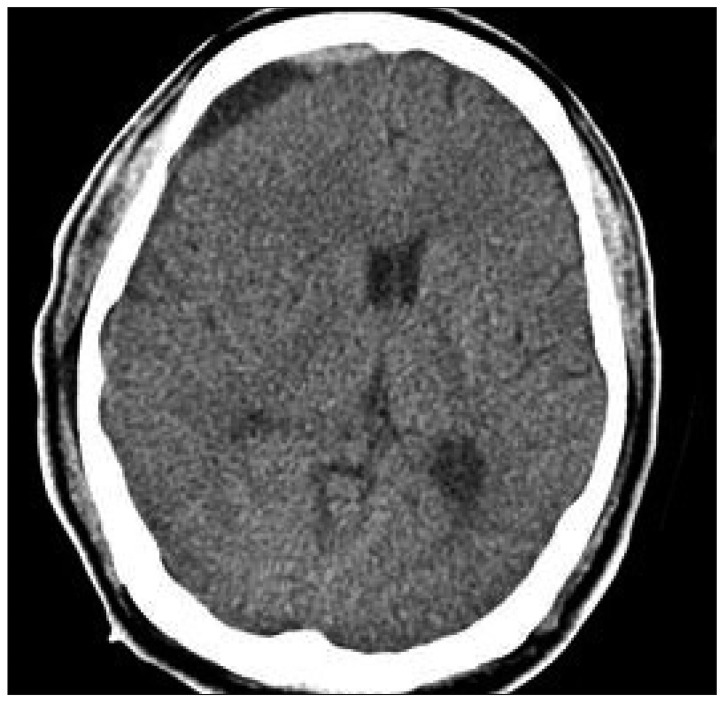
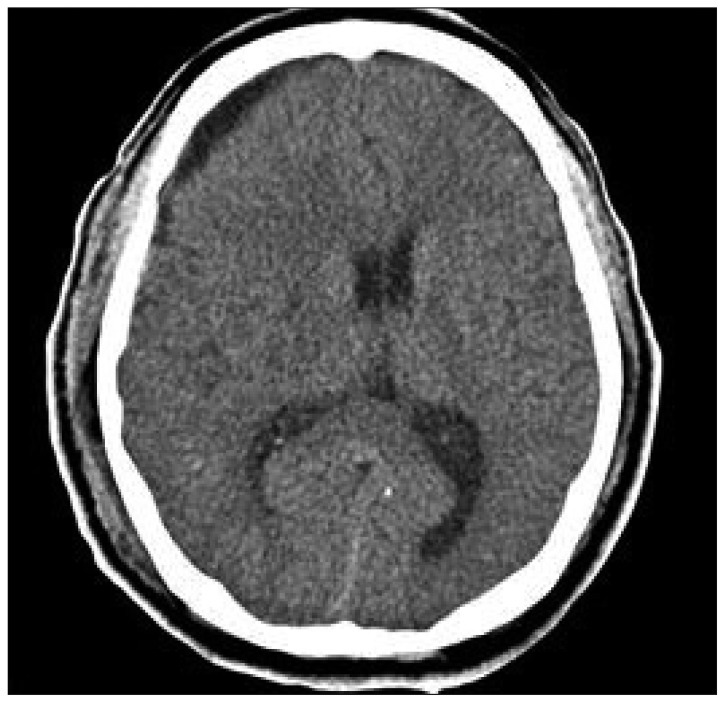
Computed tomography scan performed at the following day after revision surgery shows complete resolution of hematoma without midline shifting.
DISCUSSION
Evacuation of a chronic SDH by burr hole drainage using a 5 L-catheter is an effective and minimally invasive technique. Although the prognosis of chronic SDH is relatively good, some unusual and devastating complications may occur, such as, subdural hematoma, infection, seizure, hydrocephalus, and failure of the brain to expand, however, postoperative ICH is rare8,9,11). Intracranial hematoma after chronic SDH removal has been recently reported to be a rare but near devastating postoperative complication3,12). However, most patients with this complication develop an ipsilateral acute subdural hematoma, and massive ICH caused by coagulopathy after drainage of chronic SDH has not been previously reported. In our patient, laboratory tests for clotting profiles revealed aggravated DIC at 8 days after trephination. DIC, whether acute or chronic is usually associated with an underlying causative condition. Some diseases and conditions can disrupt the body's normal blood clotting system and lead to DIC. They include sepsis, cancer and massive tissue injury (surgery and trauma). In this case, DIC presented with no detectable preoperative underlying cause except aspirin intake and chronic SDH. Mori and Maeda10) reported the surgical results of 500 consecutive patients with chornic SDH treated by burr hole craniotomy with closed drainage system. In his study, three patients (0.6%) died due to DIC and he insisted coagulopathy was an ominous indicator of poor prognosis in patients with chronic SDH. The loss of equilibrium among the tightly regulated coagulation factors can lead either to hypercoagulable states with microthrombosis and ischemia or to hypocoagulable states with possible progression of hemorrhagic lesions after traumatic brain injury. Kawakami et al.5) also reported marked reductions of factors II, V, VII and X, and antithrombin III, and an increase in fibrinopeptide A in hematomas compared with venous blood in 19 patients. These results strongly suggest that coagulation is excessively activated regulatory mechanisms for coagulation and fibrinolysis are depressed in chronic SDH. Modesti et al.9) undertook a detailed clinical review of this entity, reporting a 5% incidence of intracerebral hematomas among 140 surgically treated patients with chronic extracerebral fluid collections. They noticed no initial abnormality in clotting profiles, including bleeding time, PT, aPTT, and platelets, in their series, as was observed in our patient.
The pathophysiologic mechanisms of intracranial hematomas occurring after evacuation of chronic extracerebral fluid collections are unclear. Many pathological events may contribute to the development of an ICH after the evacuation of a chronic subdural hematoma. For example, damage to the cerebral vasculature secondary to perioperative parenchymal shift, a sudden increase in blood flow combined with defective vascular autoregulation, and hemorrhage into a previously undetected contusion have been proposed to explain the occurrence of delayed intracranial hematomas or SDH1,2,7). This mechanism is supported by several pathologic findings. Focal cerebral edema beneath the compressed surface of the brain due to impeded venous drainage, can reduce cerebral blood flow in the affected hemisphere. Chronic dilatation of small arterial vessels and loss of carbon dioxide reactivity in the ischemic hemisphere could also contribute to the pathogenesis6). As a matter of course, aggravated coagulopathy might exert a baneful influence on recurrence of chronic SDH and intracerebral hematoma. To avoid this catastrophic complication, gradual drainage under a closed system and the effort to prevent the coagulopathy are mandatory. Some authors have proposed that twist drill holes with closed drainage provide the safest and most effective means in the presence of a chronic extracerebral fluid collection4). This procedure is effective and provides complete decompression with gradual brain re-expansion, and can also ameliorate rapid dynamic intracranial changes. Although delayed intracerebral hemorrhage after drainage of a chronic SDH is a rare complication, it can occur, as demonstrated by our case. It is not easy to predict the possibility of postoperative intracerebral hemorrhage, especially when initial coagulation parameters are normal, and thus, it is important to monitor the patients intensively after surgical evacuation of hematoma.
CONCLUSION
Although ICH caused by coagulopathy following drainage of a recurrent chronic SDH is an unusual complication, the possibility of ICH must be considered when neurologic status deteriorates. The effort to prevent coagulopathy and a slow drainage system appear to be mandatory to prevent this catastrophic complication.