Rat Peripheral Nerve Regeneration Using Nerve Guidance Channel by Porcine Small Intestinal Submucosa
Article information
Abstract
Objective
In order to develop a novel nerve guidance channel using porcine small intestinal submucosa (SIS) for nerve regeneration, we investigated the possibility of SIS, a tissue consisting of acellular collagen material without cellular immunogenicity, and containing many kinds of growth factors, as a natural material with a new bioactive functionality.
Methods
Left sciatic nerves were cut 5 mm in length, in 14 Sprague-Dawley rats. Grafts between the cut nerve ends were performed with a silicone tube (Silicon group, n=7) and rolled porcine SIS (SIS group, n=7). All rats underwent a motor function test and an electromyography (EMG) study on 4 and 10 weeks after grafting. After last EMG studies, the grafts, including proximal and distal nerve segments, were retrieved for histological analysis.
Results
Foot ulcers, due to hypesthesia, were fewer in SIS group than in Silicon group. The run time tests for motor function study were 2.67 seconds in Silicon group and 5.92 seconds in SIS group. Rats in SIS group showed a better EMG response for distal motor latency and amplitude than in Silicon group. Histologically, all grafts contained some axons and myelination. However, the number of axons and the degree of myelination were significantly higher in SIS group than Silicon group.
Conclusion
These results show that the porcine SIS was an excellent option as a natural biomaterial for peripheral nerve regeneration since this material contains many kinds of nerve growth factors. Furthermore, it could be used as a biocompatible barrier covering neural tissue.
INTRODUCTION
Various problems emerge when performing autologous nerve graft for peripheral nerve regeneration, such as donor site bleeding, pain, infection and functional loss of donor site nerve. Due to these problems, recently, when the nerve defect is largely ranged in the space between the defected nerves, a tube-shape conduit, made by various kinds of natural and synthetic materials, has been used for the purpose as nerve guidance channel5,11,13-17,20,29,30).
One of the important biomaterials is small intestinal submucosa (SIS). Badylak et al.1) have implanted the acellular biomaterial in submucosa of small intestine to various kinds of tissue for repair with excellent results. SIS implants were rapidly resorbable, supported early neovascularization, and acted as a scaffold for tissue remodeling. Thereby, they were used for regenerations of musculoskeletal system, Achiles tendon1,10), skin, abdominal wall19), dura mater2), bladder4,9,18,25) and blood vessels22,28). SIS is a natural material, having acellular collagen as an active ingredient and containing various cytokines and signal transmitters27). SIS is the unique in vivo site, where it contains the neurons except the brain and spinal cord, consisting of plenty of neurotransmitters and neurotrophic factors that are essential for the growth and survival of neurons; therefore, it was considered as they played an important role in nerve regeneration when the tissues were used as nerve guidance channels.
In this study, we developed the novel nerve guidance channel using the SIS for peripheral nerve regeneration. Sciatic nerve regeneration of Sprague-Dawley (SD) rats, using rolled SIS tube, was evaluated by run time test for motor function, electromyography (EMG) assessment and histological staining.
MATERIALS AND METHODS
Harvest of SIS
Porcine jejunum was harvested and SIS was taken in accordance with the method of Badylak et al.1) and Kropp et al.18). Large Yorkshire pig was put to death, and its entire small intestine was taken out within 1 hour postmortem. The small intestine was washed several times using a saline solution. Then, a part of the small intestine, approximately 10 cm in length, was excised and all mesenteric tissues were removed. This intestine segment was flipped, inside out, and mucosal epithelium and lamina propria were removed by rubbing carefully. After flipping it back to its original shape, tunica serosa and tunica muscularis externa were removed (Fig. 1). The SIS, which was prepared through aforementioned procedure, was sterilized with 0.1% peracetic acid and stored it at -80℃ until use.
Preparation of SIS extracts
In order to investigate the effects of growth factors in SIS on the growth and differentiation of neurons, SIS extracts were prepared. Cryopreserved SIS was divided into small pieces and they were grinded into a powder, as frozen under liquid nitrogen, using a freeze-powdering machine, the freezer mill (SPEX 6750, Metuchen, NJ, USA). The SIS powder was suspended in a 5% phosphate buffered saline, extracted sufficiently by homogenizer in ice bath. SIS solution was centrifuged at 1000 rpm for 4 minutes to remove insoluble materials. SIS extract was filtered by a syringe filter (0.22 µm, MILLEX-GV; MILLIPORE, France). Then, this solution could be used immediately, or stored at -80℃ until use.
Culture of pheochromocytoma cell (PC)
In order to investigate the presence of nerve growth factor in SIS, we performed the experiment with cultured pheochromocytoma cell (PC). The PC strain (PC-12 cell, KCLB 21721, Korea Cell Line Bank, Korea) was put into a DMEM broth (Dulbecco's modified Eagle's medium, Gibco laboratories, Rockville, MD, USA) and added L-glutamine, 15% inactivated donor calf serum (Gibco laboratories, Rockville, MD, USA), and antibiotic-antifungal agents (penicillin 100 U/mL, streptomycin 100 cgm/mL, and amphotericin B 0.25 µg/mL; Gibco Laboratories, Rockville, MD, USA). The broth prepared was placed in polystyrene flask, and the cell culture was conducted in 37℃ Incubator, containing 5% CO2.
The culturing was conducted with cells at concentration, 4×104 cells/cm3, to observe whether PC-12 cells differentiate into neurons. The culture groups were divided into 4 groups depending on the culture condition. The control group; nothing was added, the nerve growth factor (NGF) group; 10 ng/mL of NGF (NGF-7S, Mouse; Sigma chem. Co., St. Louis, MO, USA) was added, 0.5% SIS group; 0.5% SIS extracts was added, 2013and 5% SIS group; 5% SIS extracts was added. At day 1, day 2 and day 4 of culturing, we observed the neurite formation and measured the length of neurite growth, respectively, by using a microscope.
Preparation of nerve guidance channel using SIS
To produce the nerve guidance channel, SIS was excised longitudinally, and the excised section was spread and cut in the size of 7×10 cm. It was spreaded on a plastic place and dried in flattened paper shape for 6 hours at the room temperature, then rolled for making the SIS sheet. The dried SIS sheet roll was wrapped around a metal rod in 1.5 mm diameter and covered it in a tube shape. The tube shape was made by closing the bilateral sides in use of absorbable poly-glycolic acid suture (Fig. 2). The wall thickness of SIS tube was 235±23 µm. SIS tube was cut into lengths of approximately 7 mm for the sciatic nerve implantation in rats. The excised part was sterilized by using ethylene oxide gas at 30℃, and vacuum-stored until its use.
Surgical procedure
SD rats each weighing about 200-300 g were used in this study. Surgical procedures were performed under intraperitoneal ketamine (90 mg/kg) and xylazine (10 mg/kg) anesthesia. The left rear leg was shaved and sterilized, and then skin incision and muscle dissection was made for exposing the left sciatic nerve. A 5 mm of exposed sciatic nerve was resected by a knife (Fig. 3). In Silicon group (7), silicone tubes about 7 mm long were placed and sutured, using 9-0 nylon thread; whereas, in SIS group (7), previously prepared SIS tubes in length of about 7 mm were placed and sutured in the same manner, then the incision site was sutured.
Evaluation of motor function
A run time apparatus that is a specially manufactured motor assessment apparatus was used to evaluate the motor function of rats with injured sciatic nerves (Fig. 4). This apparatus is divided into 3 composition parts, which are a rotation rate regulator, motor part and rotary-motoring cylinder in diameter of 20 cm, in length of 20 cm. The rats were mounted on this apparatus, and the time, that rats could stay on it without falling, was measured and evaluated the movement of left rear leg as rotating the cylinder at a certain speed.
Electromyographic assessment
At 4 weeks and 10 weeks after grafting, the EMG assessment was performed under intraperitoneal anesthesia. One electrode was placed on peroneus longus muscle and the others were placed on proximal and distal sciatic nerves of grafting site. Under the stimulation, distal motor latency, motor conduction velocity, amplitude and area of EMG were checked.
Gross observations
At 4 weeks and 10 weeks after grafting, the presence and extent of foot ulcer, due to a loss of sensation caused by the left sciatic nerve injury, on the posterior legs were checked. Under intraperitoneal anesthesia, sciatic nerve exposure was performed on the previous surgery site of the left posterior leg. Then, gross visual observations were made on the degree of adhesion with surrounding tissues and thickness of the nerve at the graft site. After gross observation, transection biopsy of sciatic nerve was performed including the recipient site.
Histological evaluation
After EMG assessment at 10 weeks post-operatively, the nerves were resected from the recipient and the regenerated site of the left sciatic nerves. The resected nerves were fixed with 10% neutral buffered formalin. After tissues fixation, embedding was done by using a paraffin, and the tissues were sliced in thickness of 10 µm. As much as possible, this process was performed using the cross-sectional plane from the recipient site, which was obtained by cutting off from the median part of implanted nerve tissues. The transected tissues were stained with hematoxylin and eosin (H&E) dye and observed using a microscope.
For the observation with a transmission electron microscope (TEM), these tissue slices were stained with uranyl acetate and lead citrate.
Statistical analysis
Statistical analysis was performed by Student's t-test (independent-difference). Results were considered significant at p<0.05.
RESULTS
Neurite formation of PC-12 cells
During cell culture, observations were made at day 1, day 2 and day 4. The control group had shown nearly no formation of neurite from PC-12 cell (Fig. 5). The NGF had shown a lot of longer neurites. The SIS groups had shown statistically significant numbers of neurites with substantial lengths. The 5% SIS group had more number of neurites formed with longer in length, compared to the NGF group; but there was no statistical difference from the NGF group. The 5% SIS group had shown the largest number of and longest neurites grown from PC-12 cell, followed by NGF group, and the 0.5% SIS group with in the order.

A : Effects of small intestinal submucosa (SIS) extract and nerve growth factor (NGF) on PC-12 cells. The left column is the first day culture, the middle is the second and the right is the fourth. The first row is the control group, the second is the NGF group (NGF 10 ng/mL), the third is 5% SIS extract (5-SIS) and the fourth is 0.5% SIS extract (0.5-SIS). The neurites of PC-12 cells formed much more on the NGF and SIS extract containing medium than the control (×400 magnifications). B : Effects of SIS extract and NGF on PC-12 cells (*p<0.05 compared with control). The number and length of PC-12 cell neurites were in the order of 5% SIS extract>NGF>0.5% SIS extract>control.
Evaluation of sensory and motor functions
Foot ulcer had developed from a loss of sensation caused by the left sciatic nerve injury (Fig. 6). When observed after 4 weeks from grafting, 5 of 7 in Silicon group and 2 of 7 in SIS group had developed foot ulcer. After 10 weeks from grafting, 2 in Silicon group had been found with foot ulcer, but there was no incidence in SIS group. The sizes of foot ulcer were smaller in SIS group than Silicon group.
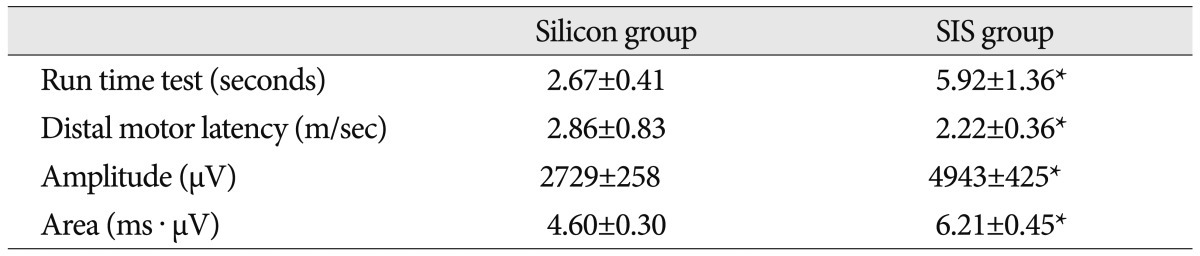
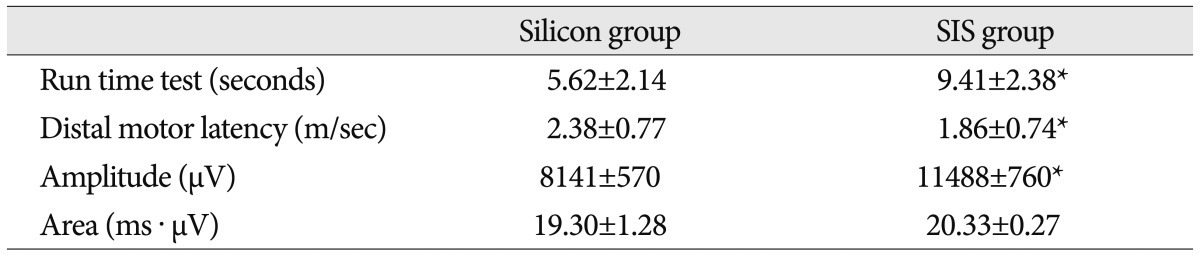
In a motor assessment test, the duration that the rats stayed and still moved on the run time apparatus, was measured as 2.67 seconds in Silicon group and 5.92 seconds in SIS group, at 4 weeks after grafting (Table 1). The movement time was far longer in SIS group (9.41 sec) than Silicon group (5.62 sec), at 10 weeks after grafting. SIS group had shown almost the same motor time of healthy rat (Table 2). During the motor evaluation, the rats in SIS group were able to partially support the body weight with the left posterior leg and move from all major joints of the left posterior leg.
Results of EMG
At week 4 and week 10 after grafting, EMG assessment was performed (Table 1, 2). The distal motor latency demonstrated at week 4 was 2.86±0.83 m/sec in Silicon group and 2.22±0.36 m/sec in SIS group, whereas, the results at week 10 after grafting had shown latency of 2.38±0.77 m/sec in Silicon group and 1.86±0.74 m/sec in SIS group, which also demonstrated better reaction in SIS group (p<0.05). Amplitude at week 4 and week 10 after the grafting and area of EMG at week 4 after the grafting had shown better EMG results in SIS group than in Silicon group, as well (p<0.05). The area of EMG at week 10 after the grafting had shown much better results, in terms of numeric outcome in SIS group; however, there was no statistical significance (p>0.05).
Gross observation and histological evaluation
At 10 weeks after grafting, the left sciatic nerves were excised and observed grossly. Silicon group had shown severe adhesion at the implanted site around the silicone, and the nerves were connected through the silicon tube. SIS group had shown complete biodegradation at week 10 and resorbed to ambient tissues; therefore, it was not possible to observe SIS, and the nerve gap considered as the bilateral ends of resection was completely filled with newly formed tissues, and the shape had been restored to almost the normal nerve.
H&E staining was performed on the cross-sectional plane taken from the median part of the implanted nerve site, and it was observed under a microscope. From the microscopic findings, SIS group has shown more number of myelinated nerve fibers, thicker axons and thicker myelin sheath, compared to Silicon group (Fig. 7).

A : Longitudinal section of nerves (Silicon group, ×200 magnifications). B : Longitudinal section of nerves [small intestinal submucosa (SIS) group, ×200 magnifications]. Multiple small cross striations of nerves are Ranvier's nodes of myelinated axons. The photograph on the left (Silicon group) shows about 10% of myelinated axons but the one on the right (SIS group) shows nearly 100% of myelinated axons. C : Cross section of silicon implanted nerve (Silicon group, ×40 magnifications). D : Cross section of SIS implanted nerve (SIS group, ×40 magnifications). SIS group (right photograph) shows more cells and vessels than Silicon group (left photograph, Silicon group). Hematoxylin and eosin staining.
Also, from the observational findings with a TEM, regeneration of myelinated nerve fibers was observed better in SIS group (Fig. 8).
DISCUSSION
The best treatment for injury of peripheral nerve is to transect the damaged part and to perform a direct anastomosis. However, if the area of injured nerve is quite large, a tensile force acts when connecting long sites, making it hard to accomplish nerve regeneration appropriately. Therefore, in such case, autologous nerve graft is used, mostly utilizing sural nerve, superficial sensory radial nerve and antebrachial cutaneous nerve. Autologous nerve graft is known as the most effective repair method so far, but it has problems in the process of collecting nerves to graft, including bleeding, infection, pain, functional loss of graft nerve and nerve injury of donor site during the harvest. Lately, owing to advancement of tissue engineering and biomedical implants, the nerve guidance channel has been developed by using various materials, alternative to autologous nerve graft.
The ideal adequate conditions, as nerve guidance channel, are that it should be able to help axonal sprouting at the proximal nerve end, work as a canal for diffusion of neurotrophic factors being secreted from the injured nerve end, should be easy to suture, prevent the tension at the suture site, and prevent the ingrowth of scar tissues through protection against the infiltration of neighboring fibers; therefore, ultimately it should be able to have positive effects on nerve regeneration. Artificially synthesized products include substances like poly-glycolic acid, poly-lactic acid and poly-lactide-co-glycolide, as well as ε-caprolactone and polyurethane based materials3,6,7,12,21). Nerve guidance channel made of natural materials are polymers consisted of collagen and hyaluronic acid8,26). All of these materials have been gradually developed with advantages and disadvantages, nevertheless, they are less effective for nerve regeneration in comparison to autologous nerve graft. In practice, they are used in part when autologous nerve graft is not performable or the length is short.
SIS is composed mainly with collagen that is natural biodegradable tissue, so that it has been used for various kinds of tissue defects because of its advantage to be degraded naturally and absorbed to surrounding tissues1). Voytik-Harbin et al.27) assumed that there are various substances and several kinds of growth factors, originated from submucous Meissner's plexus distributed in SIS. The representatives include extracellular matrix, such as fibronectin, glycosaminoglycans, heparin, heparin sulfate, hyaluronic acid, and chondroitin sulfate, in addition to collagen type I, III, IV, V and VI, as well as basic fibroblast growth factors, transforming growth factors, vascular endothelial cell growth factors, and NGF. In addition, SIS is an acellular biomaterial and does not cause any cellular immune reactions, but has excellent permeability against water, in comparison to any other materials23,24). Because of these advantages, the author used SIS to evaluate the possibility of substituting nerve implant. In the results of neurite formation of PC-12 cells, there was no difference between SIS group and NGF group. It suggests that the SIS extracts contains factors facilitating the nerve growth. Based on these results, the author had produced the nerve guidance channel with SIS for autologous nerve graft. SIS tube had shown more excellent effects of nerve regeneration than silicone tube.
In the results of EMG assessment, SIS group showed better reaction than Silicon group in the distal motor latency indicating the extent of myelin sheath formation. At week 4, SIS group showed more increase than Silicon group in the area of EMG, that presents the quantity of compound muscle action potential, an indicator of numbers of regenerated axons. Such results indicated significantly higher motor values in SIS group from the run time test that assesses the function of motor nerves, in comparison to Silicon group. And, these indicated better results in respect to the formation of ulcer, due to sensory decrease at posterior leg, in comparison to Silicon group.
Histological tests also showed SIS group had far better outcomes in formation of myelin sheath, as well as in vascular endothelial growth within the nerves of implanted site, compared to Silicon group. These results show that SIS nerve guidance channel can be used for nerve regeneration.
It is well known that rat have better regeneration ability for peripheral nerves than that of human. In the present study, 5 mm of nerve defect area was connected with silicone tube and histological test showed nerve regeneration with formation of myelin sheath, although less than 10%. Furthermore, there was the improvement of foot ulcer at 10 weeks due to the improvement of sensory nerve. These results indicated that further studies for longer nerve defect and bigger animal such as dogs and monkeys will be necessary.
For clinical application, it is considered that a comparative study to the autologous nerve graft and safety studies because the origin of SIS was porcine, not human. However, in consideration of the fact that the porcine SIS-utilized dura mater substitute (Durasis®, Cook Biotech, West Lafayette, IN, USA) is being used currently in clinical practice, after being approved by the U.S. Food and Drug Administration in terms of such xenograft-related safety, it would be all right to consider that a lot of issues were resolved.
Based on aforementioned results, the porcine SIS, as natural bio-tissue, has outstanding effectiveness in the peripheral nerve regeneration. Moreover, as it contains many kinds of NGFs and can work as biocompatible barrier to surrounding tissues so that it can be used as proper graft material for peripheral nerve injury repair.
CONCLUSION
SIS had triggered and facilitated nerve regeneration from PC-12 cell, presenting the same effects to the NGF and when implanted to the sciatic nerves of SD rats in tube-shaped form, it demonstrated outstanding induction of nerve regeneration. Therefore, it is considered that such SIS will be an effective material that can substitute autologous nerve graft in future clinical settings.