Spinal Hemangiopericytoma Which Needed Intraoperative Embolization due to Unexpected Bleeding
Article information
Abstract
Spinal intradural hemangiopericytoma is a very rare tumor and can be characterized by massive bleeding during surgeries, frequent recurrence, and metastasis. However, definite radiologic differential points of hemangiopericytoma are not known. We describe an unexpected hemangiopericytoma case with large bleeding and management of the tumor. A 21-year-old man visited complaining of progressive neck pain and tingling sensation in both hands. Magnetic resonance imaging of his spine revealed C1-2 ventral intradural mass. When the dura was opened, the intradural tumor was placed behind spinal accessary nerves. The tumor was partially exposed only after some accessary nerves had been cut. When internal debulking was performing, unexpected bleeding was noted and it was difficult to control because of narrow surgical field and hypervascularity. Intraoperative spinal angiography and embolization were performed. The tumor was completely removed after embolization. Pathological diagnosis was consistent with hemangiopericytoma. When surgeons meet a flesh-red tumor that bleeds unexpectedly during surgery, hemangiopericytoma may be considered. When feeder control is hard due to reciprocal location of spinal cord, the tumor, and feeders, intraoperative angiography and embolization may be a possible option.
INTRODUCTION
Hemangiopericytoma is a highly vascularized, aggressive neoplasm that is classified as a mesenchymal nonmeningothelial tumor with uncertain malignant potential or borderline malignancy. This tumor develops in the pericytes of soft tissue and is usually located in subcutaneous tissue and skeletal muscle. Hemangiopericytomas of the central nervous system are rare, comprising 2% to 4% of all meningeal tumors7,8). Hemangiopericytoma rarely occurs in the spine, with only approximately 60 previously reported cases of which 10 were located in the intradural extramedullary (IDEM) region1-3,5,9,10,12,14).
Hemangiopericytomas sometimes look like aggressive hemangiomas such as arteriovenous shunting or congested spinal vessels5). Local recurrence and metastasis of hemangiopericytoma were reported to 60% and 23%, respectively11). Therefore, differential diagnosis between hemangiopericytoma and other benign tumors is very important. Radiologic images of hemangiopericytoma typically shows a diffusely enhancing tumor with a broad-based meningeal attachment, which is also observed in meningioma13). A few characteristics of hemangiopericytoma were known15). However, they are not definite and are also observed in aggressive meningioma. Here, we describe a patient with an unexpected intradural hemangiopericytoma in the cervical spine, and we describe an unexpected hemangiopericytoma case with large bleeding and management of the tumor.
CASE REPORT
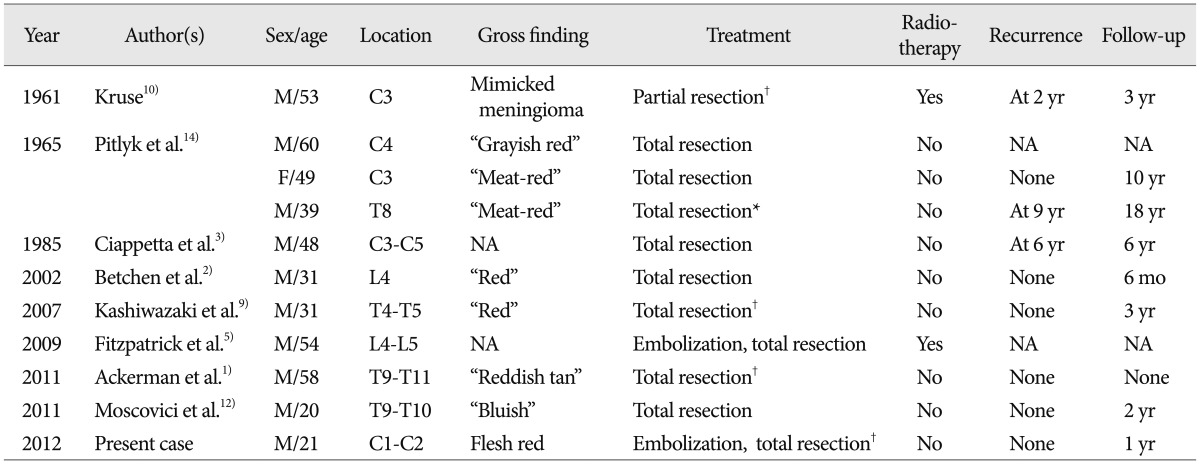
A 21-year-old man visited complaining of neck pain and a tingling sensation in both hands. The intensity of neck pain had progressed over 1 year. The tingling sensation in the hands had developed 1 month prior and had worsened. Motor function and reflexes did not decrease. His magnetic resonance (MR) images revealed 2.5-cm well-circumscribed ventral IDEM mass at the level of C1-2 (Fig. 1). The mass showed iso-signal intensity on T1-weighted images and high signal intensity on T2-weighted images. The lesion was homogenous and vividly enhanced with intravenous administration of gadolinium. Small cystic components were seen on T2-weighted images. The tumor did not expand or erode spinal canal or soft tissue. The tumor did not reveal calcification, adjacent bone erosion, and multilobulated lesions. No additional lesions were identified including brain, abdomen, and pelvis. Our presumptive diagnosis at preoperative state was meningioma, but schwannoma, and neurofibroma were also considered.
Preoperative sagittal (A) and axial (B) T2-weighted magnetic resonance images reveal a heterogeneous ventral intradural 2.5 cm diameter lesion in the C1-2 region. Sagittal T1-weighted images without enhancement (C) and with enhancement (D) reveal a well enhanced tumor with iso-signal intensity. The preoperative diagnosis was meningioma or schwannoma.
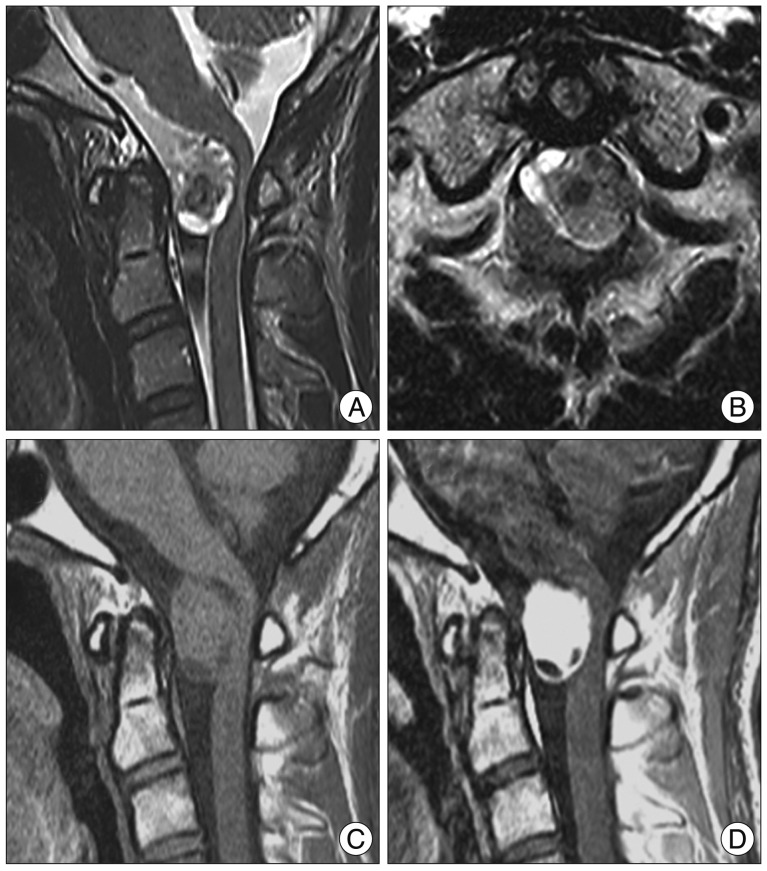
The patient underwent left hemilaminectomy at C1, left partial hemilaminectomy at C2, and partial resection of the foramen magnum. When the dura was opened, an IDEM tumor was placed behind spinal accessary nerves. The plane between the spinal cord and the mass placed well defined, and the flesh-red tumor was observed (Fig. 2). The tumor was partially exposed only after some accessary nerves had been cut. Because of narrow surgical field, we planned internal debulking first. While performing internal debulking by ultrasonic aspirator to remove the ventral IDEM tumor, unexpected tumor bleeding developed and was difficult to stop. Tumor bleeding could not be controlled by bipolar coagulator, and was stopped after compressing the lesion using microfibrillar collagen (Avitene™) for some minutes. It is difficult to find feeding arteries because of narrow surgical field. We halted the surgery and checked the spinal angiography at the angiography suite, which revealed two major feeders of the deep cervical branch from the vertebral artery (Fig. 3). Two main feeders were embolized by titanium coil or particles. After endovascular embolization of the feeder vessels, the tumor resection was resumed. The tumor was completely removed with minimal bleeding. The patient had a fast postoperative course, and the tingling sensation in his hands and his neck pain disappeared. He was discharged without symptoms and has been recurrence-free for the past 1 year (Fig. 4).

Intraoperative microscopic finding. A paramedian incision of the dura mater reveal spinal accessory nerves and an oval, flesh-red tumor (asterisk) at the ventral side. The tumor had adhered to the ventral dura. The border between the tumor and the spinal cord is well defined.
An anteroposterior (A) and lateral (B) conventional spinal angiogram demonstrates a hyper vascular tumor (arrow head) with feeding vessels (arrow). After embolization with titanium coil and particles, the tumor blush disappears on the anteroposterior (C) and lateral (D) angiogram.
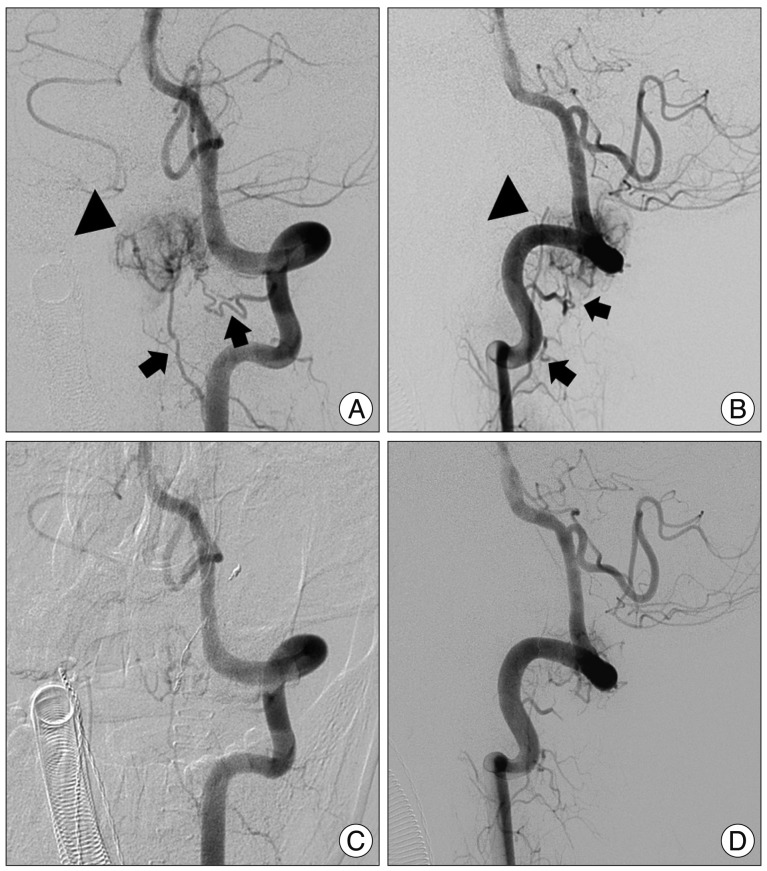
(A) Postcontrast sagittal T1-weighted image 6 months after the surgical resection demonstrates no recurred or residual tumor. Axial (B) and sagittal (C) T2-weighted images reveals that the spinal cord shifted anteriorly without the tumor. The cord is slightly diminished in caliber.
Pathologic findings
A gross specimen revealed a nodular mass of soft tissue measuring 2×2 cm. The cut surface of the mass was reddish yellow. Hematoxylin and eosin staining showed a highly cellular neoplasm growing in patternless sheets and with high mitotic activity. There was proliferation of the tumor cells with oval nuclei, and the tumor was surrounded by abundant blood vessels resulting in the "staghorn" appearance typical of hemangiopericytoma (Fig. 5). After immunostaining, the tumor cells were negative for CD34 and epithelial membrane antigen. On the basis of these findings, we made a final diagnosis of hemangiopericytoma.

(A) The tumor was somewhat firm and composed of cystic and nodular lesions. (B) Hematoxylin and eosin staining (original magnification, ×100) shows an irregular array of oval nuclei. The tumor is surrounded by many blood vessels resulting in the "staghorn" appearance typical of hemangiopericytoma. Upon immunostaining of the specimen, tumor cells are negative for CD34 (C) and EMA (D). EMA : epithelial membrane antigen.
DISCUSSION
Hemangiopericytoma is distinguished from meningioma by multilobulated lesion, expanding/eroding spinal canal, large soft tissue component, or no calcification15). Ross et al.15) addressed that malignant, invasive meningioma may be very hard to distinguish from hemangiopericytoma. However, hemangiopericytomas need to be distinguished from other tumors because they sometimes bleed substantially, recur frequently, and metastases. In this case, the tumor showed no lobulation, no bony erosion, and no soft tissue invasion on MR images like meningioma. Hence, our presumptive diagnosis, meningioma or schwannoma, was wrong. Hemangiopericytoma has a chance of missing diagnosis by radiologic studies.
In the review of previous reports, 7 of 10 papers reported hemangiopericytomas were presented as red mass in surgical field in Table 1. One tumor was described as having the gross appearance of a meningioma10), and the others were not described3,5). Although hemangiopericytoma could not be distinguished by radiologic studies in preoperative state, hemangiopericytoma seems to be distinguished by the gross feature (red color) on surgical field, which means a hypervascular tumor. Surgeons had better take extra precaution and suspect hemangiopericytoma when they watch a flesh-red mass during surgeries of IDEM tumors. Hemangiopericytoma sometimes makes large bleeding, although not in all. In case of unexpected large bleeding, feeding vessels of the tumor have to be controlled first. When feeder control is hard due to reciprocal location of spinal cord, the tumor, and feeders, intraoperative angiography and embolization may be a possible option.
In intracranial hemangiopericytoma patients, adjuvant radiotherapy or radiosurgery was usually recommended6,16). In spinal hemangiopericytoma patients, the evidence of adjuvant radiotherapy or radiosurgery was not yet clear1). In 10 previously reported cases of IDEM spinal hemangiopericytoma, 1 of 2 patients who had undergone radiotherapy recurred at 2 years after treatment. Two of 7 patients who had not undergone radiotherapy showed tumor recurrence. The data on radiation for spinal intradural lesions remains unclear because of few cases. In addition, neither radiotherapy nor radiosurgery has demonstrated protection against neuroaxis4). Therefore, we recommend closely following patients with a hemangiopericytoma clinically with serial MR imaging.
CONCLUSION
Intradural spinal hemangiopericytoma is rare but need to be distinguished from other benign tumors because it is aggressive. When surgeons meet a flesh-red tumor that bleeds unexpectedly during surgery, hemangiopericytoma may be considered. When feeder control is hard due to reciprocal location of spinal cord, the tumor, and feeders, intraoperative angiography and embolization may be a possible option.