Clinical Analysis of Intracranial Hemangiopericytoma
Article information
Abstract
Objective
Intracranial hemangiopericytomas (HPCs) are rare tumors with aggressive behavior, including local recurrence and distant metastasis. We conducted this retrospective study to evaluate the efficacy of grossly total resection and adjuvant radiotherapy (RT) for these tumors.
Methods
A total of 13 patients treated for intracranial HPC from January 1995 through May 2013 were included in this retrospective study. We analyzed the clinical presentations, radiologic appearances, treatment results, and follow-up outcomes, as well as reviewed other studies.
Results
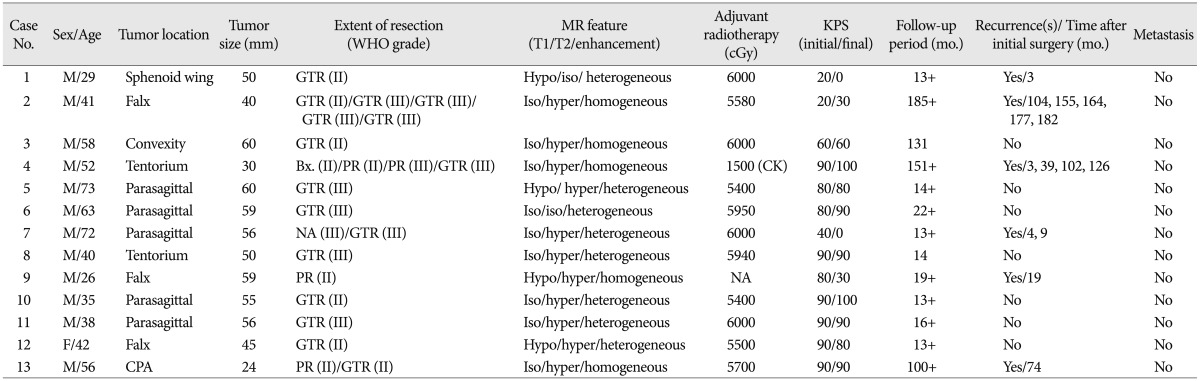
The ages of the patients at the time of diagnosis ranged from 26 to 73 years (mean : 48 years). The majority of the patients were male (92.3%), and the majority of the tumors were located in the parasagittal and falx. The ratio of intracranial HPCs to meningiomas was 13 : 598 in same period, or 2.2%. Seven patients (53.8%) had anaplastic HPCs. Nine patients (69.2%) underwent gross total tumor resection in the first operation without mortality. Eleven patients (84.6%) underwent postoperative adjuvant RT. Follow-up period ranged from 13 to 185 months (mean : 54.3 months). The local recurrence rate was 46.2% (6/13), and there were no distant metastases. The 10-year survival rate after initial surgery was 83.9%. The initial mean Karnofsky performance scale (KPS) was 70.8 and the final mean KPS was 64.6.
Conclusion
Gross total tumor resection upon initial surgery is very important. We believe that adjuvant RT is helpful even with maximal tumor resection. Molecular biologic analyses and chemotherapy studies are required to achieve better outcomes in recurrent intracranial HPCs.
INTRODUCTION
Intracranial hemangiopericytoma (HPC) was first reported by Begg and Garret4), who noted its microscopic characteristics including meningiomas. HPC arises from Zimmerman pericytes around capillaries and postcapillary venules; therefore, these tumors can occur anywhere capillaries are found, especially in the lower extremities, retroperitoneum and pelvis24,45). Intracranial HPCs rarely present in the central nervous system, and they account for 2.5% of all meningeal tumors and less than 1% of all CNS tumors22,23). These tumors also have malignant features, such as a tendency to recur locally and metastasize distantly. Despite the high rate of local recurrence and distant metastasis, there is no well formulated treatment strategy for these tumors. We reviewed our experiences with 13 patients with intracranial HPCs to analyze the disease features, treatment strategies and clinical courses. In particular, we focused on the efficacy of resection and adjuvant radiotherapy (RT).
MATERIALS AND METHODS
A total of 13 patients underwent microsurgical resection for intracranial HPC at least once at our institute from January 1995 to May 2013. The medical records of the 13 patients were reviewed and analyzed by the authors. All patients were examined using CT and enhanced MR images. These images were reviewed by an experienced neuroradiologist. We assessed tumor sizes, locations and enhancement characteristics using MR imagery. Four patients had preoperative digital subtraction angiography (DSA) and only one patient underwent tumor embolization using dimethyl sulfoxide (DMSO) and Onyx. A total of 20 operations were performed for the resection of either primary or recurrent intracranial HPCs. After 2002, all operations were performed by assisted neuronavigation. And intraoperative electrophysiologic monitoring was performed after 2009. We reviewed the original operation records to determine the estimated blood loss (EBL), macroscopic features and extent of tumor resection. The pathologic diagnoses and microscopic measuring procedures were performed by an experienced neuropathologist. Some cases were reevaluated for the purpose of this retrospective study. Immunohistochemistry was examined in all cases, including EMA, S-100, CD34 and the Ki-67 proliferative index. The mean follow-up period was 54.3 months, with a range of 13 to 185 months. Information regarding postoperative status was obtained from our outpatient department medical records and phone contacts. Statistical analysis was carried out using Kaplan-Meier method, and the comparison of recurrence free survival rate between different two groups was conducted using log-rank test. A p-value less than 0.05 was considered statistically significant.
RESULTS
Patient population
The patient group consisted of 12 (92.3%) males and 1 (7.7%) female. Patient ages at the time of initial diagnosis ranged from 26 to 73 years, with a mean of 48 years. Demographic patient data is summarized in Table 1. The patients had no history of other tumorous diseases. There were no postoperative mortalities and no unexpected events during the operations. The most common presenting symptom was headache, and four patients had visual field defects due to a tumor in the occipital lobe.
Incidence of intracranial hemangiopericytoma
During the study period, we diagnosed 13 intracranial HPCs and 598 meningiomas. The ratio of intracranial HPCs to meningiomas was 1 : 46, and the proportion of HPCs was only 2.2%.
Tumor characteristics
The preferential areas of tumor origin were parasagittal (5 patients, 38%) and falx (3 patients, 23%). One patient had a homogeneous enhancing pineal gland tumor which turned out to be HPC. Distributions of the remaining tumor locations were as follows : two convexity, one sphenoid wing and one cerebellopontine angle. The preoperative contrast-enhanced CT scans of 12 patients (CT was not available for one patient) revealed hyperdense and homogeneous or heterogeneous enhancing lesions with no evidence of calcification. Radiologic findings of osteolysis of the skull were noted in 2 patients (15.4%) (Fig. 1), but no hyperostosis was noted. In the obtained enhanced MR images, all patients had well demarcated tumor margins and detailed features of MR images are described in Table 1. Preoperative DSAs were performed on 4 patients (30.8%) because they had many fluid void signals on the T2-weighted images. Each DSA image showed a hypervascular contrast staining mass with an arterial feeder from the middle meningeal artery (Case No. 2), branches of the left PICA (Case No. 8), distal branches of the pericallosal artery and some posterior choroidal arteries (Case No. 9), left MCA, PCA and transcranial ECA (Case No. 10). Two patients (15.4%) showed hemorrhage from the HPC on the initial CT scans (Case Nos. 1, 2) (Fig. 2).
Treatment modalities
Preoperative embolization
Only one patient (Case No.10) had effective preoperative embolization of the tumor. One day preceding the operation for tumor resection, embolization was performed by DMSO and Onyx via the left distal MCA. Post-embolic angiography showed a decrease in the size of the hypervascular contrast staining mass (Fig. 3). An MR image obtained immediately after embolization of the tumor showed no acute infarction or venous congestion. Additionally, the patient had no neurological deterioration after embolization.
Surgery
All patients were initially treated with surgical resections and there were no perioperative mortalities. At the initial operation, a grossly total resection (GTR) was accomplished in 9 patients (69.2%), a partial resection (PR) in 2 patients (15.4%) and an endoscopic biopsy in 1 patient, while results were not available for one patient. Two patients had aborted operations because of intraoperative bleeding that resulted in partial resection. One patient underwent only an endoscopic biopsy because of a deep seated tumor (pineal lesion, Case No. 4). Another patient underwent the initial operation at an outside institution, so we could not estimate the extent of the resection. According to the original operation records, EBL ranged from 350 mL to 1200 mL and the mean EBL was 850 mL, for all patients. Understandably, the patient (Case No. 10) who performed preoperative embolization had the lowest EBL (350 mL).
Radiotherapy
Postoperative external beam radiotherapy (EBRT) was delivered in 11 patients (84.6%) regardless of the extent of resection. One patient (Case No. 9) rejected an active treatment after the initial surgery and another patient (Case No. 4) was not irradiated postoperatively due to misdiagnosis. However, he received irradiation (1500 cGy) by cyberknife 3 months later. The mean total EBRT dose was 5770 cGy (range : 5400-6000 cGy). Two patients (15.4%) underwent stereotactic radiosurgery using a cyberknife for treatment of recurrent tumors.
Chemotherapy
One patient (Case No.4) began chemotherapy after the initial operation. His initial pathologic diagnosis was pineal parenchymal neoplasm with intermediate undifferentiation, and he received a total of 2 cycles of ICE chemotherapy (a regimen employing ifosfamide, carboplatin and etoposide). But, the tumor progressed during chemotherapy and mental confusion developed due to an obstructed hydrocephalus. A second operation was performed 40 months after the initial operation. The final diagnosis of second operation was identified.
Pathologic results
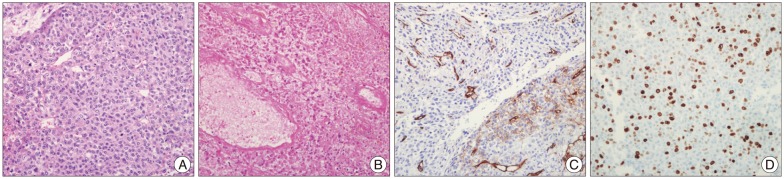
Six patients (46.1%) had low grade HPCs (WHO grade II) and 7 patients (53.9%) had anaplastic HPCs (WHO grade III), a percentage that is relatively higher than in other studies. Interestingly, the pathologic diagnosis of the resected recurrent tumors changed from low grade to anaplastic HPCs in 2 patients (15.4%, Case Nos. 2, 4). Low grade HPCs (WHO grade II) showed dense cellularity with branched thin walled vessels (Fig. 4A). Tumor cells had diffuse CD34 positivity (Fig. 4B) and the Ki-67 proliferative index was 10% (Fig. 4C). Anaplastic HPCs (WHO grade III) showed cellular pleomorphism with frequent mitosis and necrosis (Fig. 5A, B). CD34 was focally positive (Fig. 5C) and the Ki-67 proliferative index was 30% (Fig. 5D). All resected tumors had well developed reticulin fibers, and CD34 was positive in 10 patients (83.3%) and negative in 2 patients (16.7%). There was one patient whose CD34 was not checked. The Ki-67 proliferative index ranged from 1% to 30%, wi th a mean of 11.3%. The mean Ki-67 proliferative index was 2.2% in the low grade HPC group, and 18.3% in the anaplastic HPC groups.

Pathologic features of low grade hemangiopericytomas. A : Hematoxylin and eosin, ×200. B : CD34, ×200. C : Ki-67, ×200.
Local recurrences and distant metastases
Six patients (46.2%) developed local recurrences at 104, 73, 19, 4 and 3 (two patients) months after initial surgery. Of the 6 patients, one underwent five operations, another underwent four operations and the other two underwent two operations. The two remaining patients underwent only the initial operation because of their poor general medical condition. Five and 10-year recurrence-free survival (RFS) rate was 62% and 20.5%, respectively. The median RFS was 74 months (Fig. 6). On subgroup (GTR or not) analysis, mean time for local recurrence was 24.6 months in patients who treated with PR or biopsy against 105.7 months in patients who treated with GTR (p=0.012 by log-rank test) (Fig. 7). Regarding adjuvant RT, mean time for local recurrence was 10.7 months in patients who did not received RT against 85.4 months in patients who received RT (p=0.052 by log-rank test) (Fig. 8). There were no distant metastases, based on clinical follow-up and whole body PET-CT scan.
Overall outcome and survival
The outcomes of treatment were estimated according to the Karnofsky performance scale (KPS). The mean KPS at the final outcome was 64.6 (Initial mean KPS was 70.8). The mean follow-up period was 54.3 months (range : 13-185 months). Two of the 13 patients died during the follow-up period. Five year overall survival rate was 84% and mean overall survival was 158 months (Fig. 9).
DISCUSSION
Intracranial HPCs are very rare tumors with an incidence of less than 1%, and they represented 2.2% of all meningiomas diagnosed over the last 18 years in our institute. Alén et al.1) reported an incidence of intracranial HPCs similar to our data. It is widely known that intracranial HPCs, unlike meningiomas, are more common in males than females, which was also true in our data, where 92% of the patients were males5,18,25). In previous studies on intracranial HPCs, the mean age at the time of diagnosis ranged from 38 to 44.9 years, which was lower than that of meningiomas1,5,22,23,25,48). In our series, the average age was 48 years. Intracerebral hemorrhage was relatively more common in our patients than in others17,19). Our study included two patients (15.4%) with intracerebral hemorrhage on an initial CT scan. The majority of HPCs arise in the supratentorial area with dural attachment23,41). However, non-dural attached HPCs, such as in the pineal region (Case No.4), and purely intraparenchymal HPCs have also been reported28,39,41).
Distinguishing HPCs from benign meningiomas before surgery can be difficult, but is very important because of the aggressiveness of HPCs and their high rates of local recurrence and distant metastasis. Many previous papers have discussed the differential diagnosis between HPCs and meningiomas. Chiechi et al.9) reported that intracranial HPCs are more multilobulated than benign meningiomas, and have narrow based dural attachment on CT and conventional MR images. Additionally, hyperostosis and intralesional calcification are not found in intracranial HPCs, which is distinct from meningiomas. Many other papers have reported that intracranial HPCs may show osteolytic features on plain films, CT and MR images6,11,33). In the current study, osteolysis of the skull on CT scan was noted in 2 patients (Case Nos. 5, 11) who were diagnosed with anaplastic HPC. It has been found that osteolysis is more prevalent in anaplastic HPCs than in low grade HPCs9). It has also been found that CT perfusion is valuable in preoperative grading of intracranial gliomas13), in distinguishing between glioblastomas, metastasis and abscesses8,12) and in evaluating the prognosis of recurrent brain tumors after stereotactic radiosurgery26). Similarly, Ren et al.34) reported that CT perfusion can provide crucial information on HPCs and benign meningiomas. They found that HPCs showed higher blood volume than benign meningiomas. As MRI technology has advanced, Liu et al.30) demonstrated that minimum apparent diffusion coefficient (ADC) values may be helpful for distinguishing HPCs from meningiomas. They found that the mean minimum ADC values of HPCs were significantly higher than that of meningiomas.
The frailty of the conventional surgical approach was intractable intraoperative bleeding from the meningeal artery during the initial stage of the operation. Thus, several authors have proposed multi-stage operations, included feeding artery embolization27). Matsushige et al.31) reported a giant HPC treated with preoperative tumor embolization, where embolization was used to overcome intraoperative bleeding. The use of preoperative tumor embolization has also decreased operation-related mortality and morbidity1). After devascularization of the tumor from the main feeding artery using embolization, we were also able to markedly reduce intraoperative bleeding in Case No. 10. The blood loss during operation was much lower compared to non-embolized patients (350 mL vs. 990 mL), but we could not draw statistically significant conclusions due to the limited sample size of our study.
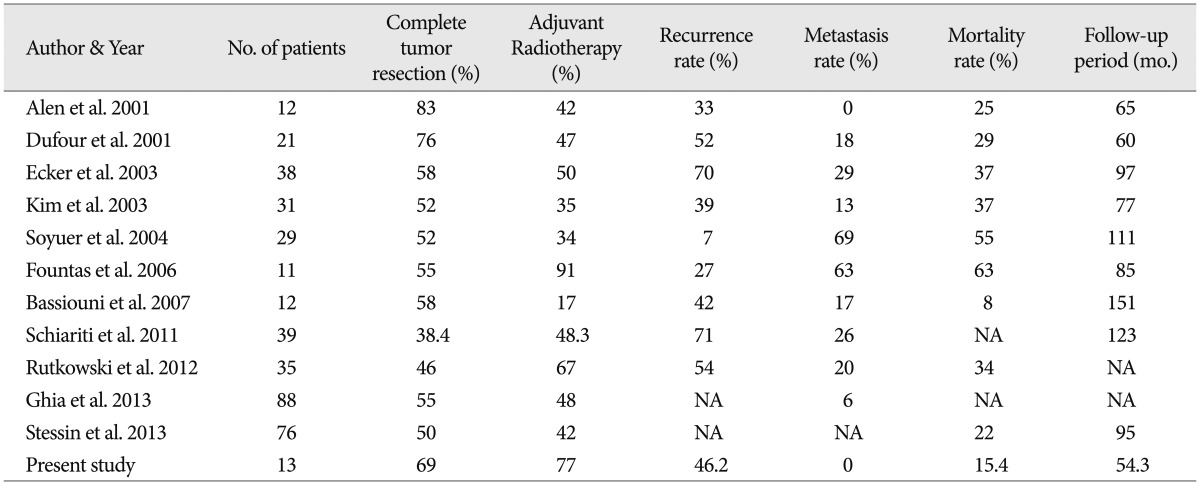
Surgical resection of the tumors offers the advantage of pathologic confirmation and reduction of mass effects. A Simpson grade I resection has been found to be associated with a high long-term control rate2,18). Similarly, Rutkowski et al.36) reported that, regardless of adjuvant therapy, GTR was associated with significantly improved survival rates compared with PR. In our series, a total of 9 patients (69%) achieved a GTR at the initial surgery, which was a much higher rate compared to other studies. A literature review on intracranial HPCs showed an average GTR rate of 56.7% (Table 2). We conducted a statistical analysis about correlation between extent of resection and recurrence, and we found statistical significance like other several series (mean RFS 105.7 months vs. 24.6 months, p=0.012) (Fig. 7)2,18). Interestingly, the average mortality rate in the recent literature was 31.5%1,2,15,16,18,21,28,35,37,42,44), but was only 15.4% in our study. Though adjuvant therapy and follow-up period also affect the mortality rate, we cautiously suggest that GTR also contributed to the decreased mortality rate.
Intracranial HPCs have been shown to commonly recur even after complete GTR. Thus, several authors have recommended adjuvant treatments for better outcomes1,5,10,15,16,18,23,32,40). Staples et al.43) reported that the 5-year RFS improved from 28% with operation alone to 57% with adjuvant RT after GTR. Similarly, we could find that the patients who had been recieved adjuvant RT had tendency of longer RFS (mean RFS 85.4 months vs. 10.7 months, p=0.052) (Fig. 8). A review of the recent literature shows that adjuvant RT is not applied consistently (range : 17-91%, mean : 47.4%). On the other hand, we performed adjuvant RT regardless of the pathologic grade and extent of resection in most of the patients, 11 out of 13 patients received adjuvant RT to a mean dose of 5770 cGy. Serious side effects from irradiation, such as cerebral edema, leucencephalopathy, radiation-induced necrosis and death have been reported42). In our study, there were no significant radiation-related complications.
Chemotherapy provided only insignificant benefits3,20), but Chamberlain and Glantz7) reported that chemotherapy (cyclophosphamide+adriamycin+vincristine, followed by α-IFN, followed by ICE) may be helpful for recurrent intractable intracranial HPCs. In our study, one patient was treated with chemotherapy (ICE regimen) due to an initial misdiagnosis of pineal sarcoma. Unfortunately, the results of chemotherapy were not effective.
The histological criteria for anaplastic HPCs (WHO grade III) include more than five mitoses in 10 high power fields and/or necrosis and moderate to high nuclear atypia and moderate to high cellularity after hemorrhage. Moreover, the pathologic features of low grade HPCs (WHO grade II) show monomorphous round to oval tumor cells having staghorn-like vessels. The tumors contain well developed reticulin fibers and diffuse CD34 immunopositivity. In our data, the mean Ki-67 proliferative indexes in low grade and anaplastic HPC were 2.2% and 18.3%, respectively. In recurrent HPC, the mean Ki-67 was 14.6%, while in non-recurrent HPC, it was 7.8%. However, the number of cases was too small for statistical significance. Vuorinen et al. reported that the patients with a Ki-67 index less than 5% tended to survive longer, but this finding could not correlate the clinical outcome46,47). Also we conducted statistical analysis of RFS depending on the WHO grade, but we could not find statistical significance (p=0.644).
The majority of HPCs recur at the primary site. Galanis et al.20) found that, out of 32 recurrences, 19 (59%) recurred at the primary site, eleven (34%) recurred at both the primary and other CNS sites and the remaining 2 (7%) had diffuse leptomeningeal spread without local recurrence at the primary site. In our series, all local recurrence occurred at the primary site. We would define 'recurrence' as increased tumor sized by follow-up radiologic study and any apparent deterioration in clinical status to need reoperation. In the strict sense, initially partial resection or biopsy cases should be described as 'progression' when reoperation were needed, but number of our cases were too small to analyze about progression cases, separately. Unfortunately, several authors reported that the extent of tumor resection has been less definitely correlated with the local recurrence of HPCs compared to meningiomas29). This feature of intracranial HPCs might be explained by the strategy to aggressively irradiate partially resected tumors to reduce local recurrence. Recently, Du et al.14) reported only a 3.8% recurrence rate, but their follow-up period was very limited (mean : 22 months). In our series, the recurrence rate was 46.2% (6/13), with the mean follow-up period of 54.3 months (range : 13-185 months).
It is widely known that after the first recurrence, HPC tends to recur at brief intervals. Guthrie et al.23) reported that the average periods to the second, third and fourth surgeries for recurrence were 38, 35 and 17 months, respectively. Our patients with multiple recurrences also tended to follow a similar timeline.
The 10-year survival rate in our study was 83.9%. Schröder et al.38) found 5-, 10- and 15-year survival rates of 65%, 45% and 15%, respectively. In their broad literature review, Rutkowski et al.36) found 1-, 5-, 10- and 20-year survival rates of 95%, 82%, 60% and 23%, respectively. Mena et al.32) reported a recurrence rate of 70% (57/94), while Kochanek et al.29) reported 41% (7/17). It seems that rarity of intracranial HPCs along with the absence of a well formulated treatment strategy might lead to highly fluctuating survival and local recurrence rates.
CONCLUSION
Safe and tolerable gross total tumor resection in the initial surgery is very important. We believe that adjuvant radiation therapy is helpful, even if maximal tumor resection was performed in low grade HPCs. Preoperatively, differentiation between HPCs and meningiomas is necessary for formulating a treatment strategy. Evaluation of distant metastasis through long-term follow-up is also important. Molecular biologic analysis and studies of the efficacy of chemotherapy are required for better outcomes in relentlessly recurrent HPCs.