Rapid Progression of Solitary Plasmacytoma to Multiple Myeloma in Lumbar Vertebra
Article information
Abstract
The prognosis of solitary plasmacytoma varies greatly, with some patients recovering after surgical removal or local fractional radiation therapy, and others progressing to multiple myeloma years later. Primary detection of progression to multiple myeloma is important in the treatment of solitary plasmacytoma. There have been several analyses of the risk factors involved in the early progression to multiple myeloma. We describe one case of solitary plasmacytoma of the lumbar vertebra that was treated with surgical decompression with stabilization and additional radiotherapy. The patient had no factors associated with rapid progression to multiple myeloma such as age, size, immunologic results, pathological findings, and serum free light chain ratio at the time of diagnosis. However, his condition progressed to multiple myeloma less than two months after the initial diagnosis of solitary plasmacytoma. We suggest that surgeons should be vigilant in watching for rapid progression to multiple myeloma even in case that the patient with solitary plasmacytoma has no risk factors for rapid progression to multiple myeloma.
INTRODUCTION
Solitary plasmacytoma (SP) is defined as a solitary mass of neoplastic plasma cells, and can be classified into 2 types according to location : skeletal and non-skeletal plasmacytoma. The clinical outcome of SP varies greatly; many patients are cured with the appropriate therapy but some patients develop disseminated multiple myeloma (MM) years later. Radical radiotherapy and alternative surgery are treatment modalities producing sufficient local control12,14). However, despite these treatments, 50-60% of patients with SP progresses to MM6,17). Regarding time to progression, Knobel et al.13) reported that median time to MM development from skeletal SP was 21 months with a 5-year probability of 51% and Bertanha et al.1) analyzed the average time was 41 months. Thus, identifying the predictors associated with plasma cell malignant proliferation, in addition to the primary detection of aggravation of the SP are crucial in the management and survival of patient. In general, clinicians assume that patients with SP who do not have profiles consistent with progression to multiple myeloma will be eventually cured, or will progress to MM only slowly.
This report describes a case of SP of the lumbar vertebra without progression factors, which developed into MM less than two months after initial diagnosis despite appropriate treatment. We also review previous reports on the related factors involved in disseminated MM.
CASE REPORT
A 48-year-old man visited the neurosurgery department for right leg pain. He had experienced low back pain and hyperesthesia in the right leg for six weeks without a trauma history. One week prior to presentation, he experienced right hip flexion weakness. Neurological evaluation revealed right hip flexion (G3/5) and extension weakness (G4/5), and the patient showed an abnormal increased sensitivity in the right leg L3 dermatome, and he had continuous lower back pain with tenderness.
Computed tomography (CT) scanning and magnetic resonance imaging (MRI) showed a geographic osteolytic lesion involving the right mid-posterior element of the L3 vertebra and the right psoas muscle, and an epidural mass with dural sac compression (Fig. 1A, B). Subsequently, the patient underwent F-18 FDG whole body PET/CT examination, which revealed a destructive bone lesion and a paravertebral soft tissue mass with mild increased FDG tracer uptake (SUVmax=4.6)(Fig. 1C). There was no evidence of metastasis.

Preoperative CT (A : axial) and MRI contrast-enhanced T1-weighted images (B : axial) reveal a lytic lesion involving the right mid-posterior area of the L3 vertebral body and the right psoas muscle, and an epidural mass producing dural sac compression. Preoperative whole body PET/CT (C : coronal) shows a right paravertebral soft tissue mass with mildly increased FDG-tracer uptake, and no metastases.
We performed a total corpectomy of L3 to remove the destructive column and dural sac mass and stabilized the vertebrae with an expandable interbody cage (Synex System®, SYNTHES, USA) and posterior pedicle screw fixation L1/2/4 (Varian medical system®, VARIANS, USA) (Fig. 2). The patient's neurologic symptoms were improved to normal neurologic function after surgery.

Postoperative plain X-ray after surgical decompression with stabilization (anterior to posterior) reveals expandable interbody cage and posterior pedicle screw fixation L1/2/4.
Histological diagnosis of the resected tumor was plasmacytoma. The immunohistochemical (IHC) staining of the neoplastic plasma cells revealed only weak Immunoglobulin (Ig) kappa chain restriction and CD138 positive expression (Fig. 3). Bone marrow aspiration biopsy was performed when the plasmacytoma was diagnosed, and normal marrow proliferation was observed. Serologic studies of immunofixation and protein electrophoporesis revealed weak monoclonal gammopathy, IgG-kappa type. The serum free light chain (SFLC) ratio was 1.16 (normal reference range; 0.26-1.65). The patient had no rapid progression factors and underwent local fractional radiotherapy (RT) at a dose of 45 Gy.

Postoperative immunohistochemical staining of resected mass (A : Immunoglobulin restriction, B : CD138) shows Ig Kappa chain restriction and CD138 positive reaction.
Less than two months after pathologic diagnosis, he noted a painful, soft mass, 3×3 cm in diameter in the left wrist. There was also a palpable right inguinal mass. A repeat PET/CT also demonstrated multiple bony masses with moderately increased FDG uptake in the left distal ulna, right parietal skull, right and left clavicle, right and left humerus, right radius, sternum, left 8th rib, right and left ilium, left acetabulum, and left proximal femur, due to spread of malignant tumor. A soft tissue mass with increased FDG uptake was noted in the right proximal thigh with lymph node enlargement seen in the right and left external iliac, left pericolic, and right inguinal lymph nodes (Fig. 4). The right inguinal mass was excised for evaluation for multiple myeloma, and IHC staining of the mass revealed a strong kappa chain positive reaction. The mass was CD138 positive and there was high proliferative activity of Ki-67 detected. The SFLC ratio was elevated to 32.3.
The patient was diagnosed with MM and underwent adjuvant chemotherapy, and thalidomide and dexamethasone were administered.
DISCUSSION
Solitary plasmacytoma of bone (SPB) represents only 5% of all plasma cell malignancies and is a heterogenous condition4,5,9). Some patients have only solitary bone lesion, while others progress to MM. The usual presentation of this is with bone pain; however, 25% develop neurological dysfunction in the form of cord or nerve root compression19,21). The diagnosis of SPB requires the presence of a solitary bone lesion confirmed by skeletal survey including bone scan or PET/CT, abnormal plasma cell proliferation proven by bone marrow or tissue biopsy and lack of proof of organ dysfunction1,3,25,30).
Radiotherapy is considered the treatment of choice for SPB11). Knobel et al.13) confirmed favorable local disease control with radiotherapy alone in their review of 206 patients with SPB. Local relapse occurred in 21 (14%) out of 148 patients who received radiotherapy alone compared with 4 (80%) out of 5 patients who were treated with surgery and chemotherapy. Previous studies recommend radiotherapy for SPB encompassing the tumor volume shown on MRI with a margin of at least 2 cm and treating to a dose of 40 Gy in 20 fractions with a higher dose of 50 Gy in 25 fractions being considered for SPB>5 cm24).
Recently researchers evaluated the outcomes of over 125 patients with SP receiving only radiation therapy as initial treatment. Radiation controlled the local plasmacytoma in over 90% of the patients. Recurrences occurred in only 20% of patients who showed a disappearance of immunoglobulin (M-protein) following treatment with radiation. Patients who did not show a decrease in M-protein levels following radiation therapy had a 60% chance of recurrence. This result was compared to other reports evaluating the outcome of patients with SP who received both chemotherapy and radiation as initial treatment. The addition of chemotherapy to radiation therapy revealed no overall benefit regarding the rate of progression to MM5,6).
Surgery ("radiotherapy" versus "surgery and radiotherapy") did not affect the 10-year probability of local control5,6). Therefore, surgical resection is not indicated for SPB, but some patients may require neural decompression, spinal stabilization because of their neurologic compromise or structural instability8,17,21). This patient complained of right leg monoparesis with difficulty in ambulation and lumbar MRI revealed epidural mass with dural sac compression of L3. He underwent surgical decompression with stabilization of L1/2/4 using pedicle screws.
According to the mostly used Durie and Salmon (DS) staging system, SPB is regarded as Stage I myeloma. In 2005, the international myeloma staging system (ISS) was announced which divides DS stage I patients into three further stages9). This patient belonged to DS Stage IA and ISS Stage I; this stage is defined as showing mildly increased serum beta 2-microglobulin and normal serum albumin. The natural course of Stage I when traced over a 10-year follow-up period demonstrates four patterns of prognosis. These are development of MM (65%), local recurrence (12%), dissemination to a new solitary lesion (15%), and cured. Regarding times to progression to MM, there is a wide month range. The average median time is 24 to 36 months5,9,13,18,25), Knobel et al.13) reported it is 21 months (range : 2-135), and Bertanha et al.1) announced it is 41 months (range : 1.5-120). Less than two months, rapid progression to MM in SP of DS stage IA and ISS stage I without potential dissemination factors is a rare condition.
For the difficulty in predicting prognosis, most patients need further follow-up to detect possible progression of SP and monitoring with regular serum or urine immunologic studies. There are several reports on the potential risk factors that influence the frequency of progression to MM.
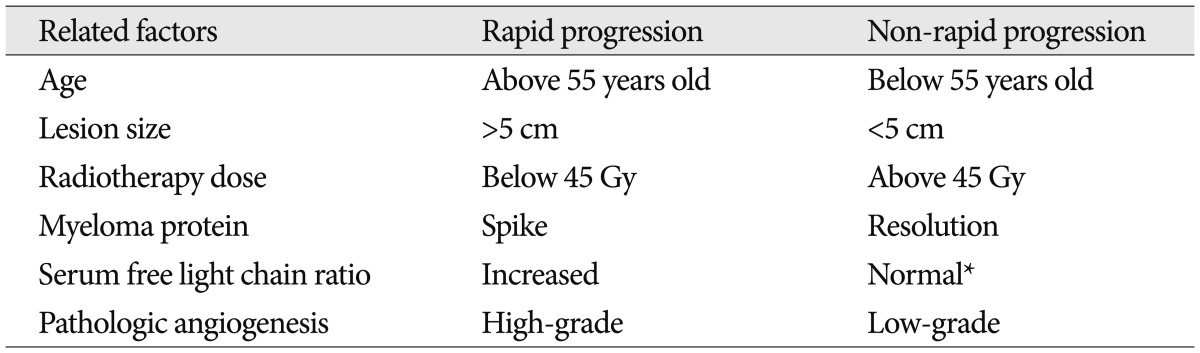
Knobel et al.13) found that younger patients, especially with vertebral localization, had the better outcome when treated with moderate-dose (30 Gy) RT. Kilciksiz et al.12) reported that younger age was an independent good prognostic factor for progression to MM. Lesion size was reported as one of the prognostic features for conversion of SPB into MM. Tsang et al.27) showed that patients with a mass size <5 cm had a local control rate of 100%, while for patients with larger tumors, the rate reached was almost 40%. In this case, the disrupted soft mass was about 4 cm in diameter. The optimum RT dose of treatment of SPB has not been established20) but local failure has not been observed in the patients who received 45 Gy or more to the isolated lesion15,27). Following RT application, persistent myeloma protein was an adverse prognostic factor. In most SPB patients, the myeloma protein level has a key role in the primary detection of MM13,16,19,29) Pathological factors have been investigated in some studies. The existence of a high level of angiogenesis is linked to a poor outcome. Kumar et al.16) examined whether increased angiogenesis may help to identify the patients likely to progress to myeloma. Recently, an abnormal SFLC ratio has been shown to be a powerful prognostic factor in determining the risk of progression to MM at the time of the initial diagnosis in patients with SP7,22,23). To assess clonality, Dingli et al.7) used the ratio of kappa-lambda light chain levels and reported that patients with an abnormal FLC had a higher incidence of monoclonal protein in the urine and a larger serum M spike. We have summarized the generally accepted early progression factors (Table 1).
The likelihood of surgery-induced dissemination may be a considerable risk factor. The surgical procedure itself reduces immunity and diminishes natural killer (NK) cell activity 2,10,26). NK cell activity falters when it is most needed to fight cancer cells. Angiogenesis is the process by which new blood vessels are formed during wound healing process after surgery. However, the new formation of blood vessels can also supply the remnant tumor. This may not only benefit local recurrence and the formation of metastatic disease, but also result in activation of dormant micrometastases16,28). In abundant angiogenesis condition, isolated plasma cell tumors that break away from the primary SPB must breach the connective tissue and then enter a blood vessel or lymphatic system28).
The patient in our case was diagnosed with SPB during his first admission. Though he had no progression risk factors, less than two months later the SPB of the lumbar vertebra had developed into MM despite appropriate treatment. We supposed that the possibility of rapid dissemination caused by surgery itself may be considered in this patient without any progression factors.
CONCLUSION
Though patients presenting with solitary plasmacytoma may have no risk factors for developing MM, we suggest that surgeons should be always be aware of the potential for rapid progression to MM from the time of the initial diagnosis.
Acknowledgements
This study was supported by the BioGreen21 Program (PJ009051) of Rural Development Administration.