Anterior Choroidal Artery Aneurysm Surgery: Ischemic Complications and Clinical Outcomes Revisited
Article information
Abstract
Objective
Surgical results for anterior choroidal artery (AChA) aneurysms have previously been reported as unsatisfactory due to inadvertent occlusion of the AChA, while the low incidence of AChA aneurysms hampers the accumulation of surgical experience. The authors reviewed their related surgical experience to document the ischemic complications and surgical outcomes.
Methods
Identification of the AChA at its origin by rigorous visual scrutiny, careful microdissection, and meticulous clip placement to avoid the AChA origin are all crucial surgical maneuvers. A retrospective review of a surgical series of 62 consecutive cases of an AChA aneurysm between 2004 and 2012 was performed.
Results
All patients, except for five (8.1%) with a small residual neck, showed complete aneurysm obliteration in postoperative angiographic evaluations. There was no incidence of procedure-related permanent AChA syndrome or oculomotor nerve palsy, while three (4.8%) patients suffered from transient AChA syndrome. The clinical outcomes [the 3-month modified Rankin Scale (mRS)] of the patients were related to their preoperative World Federation of Neurologic Surgeons (WFNS) grade. As regards the 3-month mRS, significant differences were found between patients with an unruptured aneurysm (WFNS grade 0; n=20), good-grade subarachnoid hemorrhage (WFNS grade 1-3; n=30), and poor-grade subarachnoid hemorrhage (WFNS grade 4-5; n=4).
Conclusion
In surgical treatment of AChA aneurysms, a risk of AChA insufficiency can be minimized by taking every precaution to preserve the AChA patency and intraoperative monitoring. In addition, the surgical outcome is primarily determined by the preoperative clinical state.
INTRODUCTION
An aneurysm arising from the internal carotid artery (ICA) at the origin of the anterior choroidal artery (AChA) is one of the most easily accessible surgical lesions that can be reached via a pterional or even a supraorbital keyhole approach without much difficulty. Notwithstanding, the surgical results can still be unsatisfactory due to inadvertent occlusion of the AChA, leading to a permanent AChA syndrome with an incidence of 5 to 16%1,5,6,10,17,20). Furthermore, the low incidence of AChA aneurysms has hampered the accumulation of surgical experience with infrequent publications on the surgical outcomes of AChA aneurysms.
However, recent advancements in surgical techniques and intraoperative monitoring to preserve the AChA have decreased ischemic complications and improved surgical outcomes. Accordingly, the authors reviewed a single-institution surgical experience based on 62 patients who underwent surgical clipping for an AChA aneurysm to document the ischemic complications and surgical outcomes. The surgical technique to preserve the AChA is also described in detail.
MATERIALS AND METHODS
Patients
The design of this study was a retrospective review of data from a series of 62 consecutive patients who underwent surgical clipping of an AChA aneurysm by a single surgeon (J.P.) between November 2004 and June 2012. Meanwhile, during this same period, other 12 patients with an AChA aneurysm were treated using endovascular coiling.
Information was obtained from the patient charts, surgical reports, video records, reviews of radiological investigations, and notes from follow-up visits to investigate the ischemic complications and surgical outcomes.
Operative technique
The AChA aneurysms were approached using a pterional or supraorbital keyhole approach. A detailed description of the supraorbital keyhole approach has already been published elsewhere by the authors12,13).
Frontal lobe retraction after dissecting the most proximal part of the sylvian fissure allows visualization up to the carotid bifurcation. The arachnoid is dissected along the superior wall of the ICA and rolled laterally to expose the aneurysm base and origins of the posterior communicating artery (PCoA) and AChA. A few small vessels arising from the ICA can be observed and should be preserved as they can also supply the AChA territory.
Determining the exact origin of the AChA is crucial and can only be confirmed by rigorous visual scrutiny under high microscopic magnification. The origin of the AChA is most commonly located at the inferior or posterior aspect of the aneurysm. When the AChA origin is posterior to the aneurysm obscuring the artery from view, the aneurysm can be compressed and manipulated using a blunt dissector to reveal the hidden AChA origin after softening the aneurysm by temporary ICA clipping. In cases of a ruptured aneurysm, tentative clipping at the rupture point or trapping the aneurysm using proximal and distal ICA clips can facilitate dissection and manipulation of the aneurysm.
When placing the aneurysm clips, utmost effort must be made to avoid compromising the AChA origin. If the AChA arises inferior to the aneurysm, a clip is applied perpendicular to the axis of the ICA. The proximal blade of the clip is positioned a small distance away from the origin of the AChA so as not to constrict the AChA origin, while the distal blade is positioned nuzzled close to the ICA (Fig. 1).
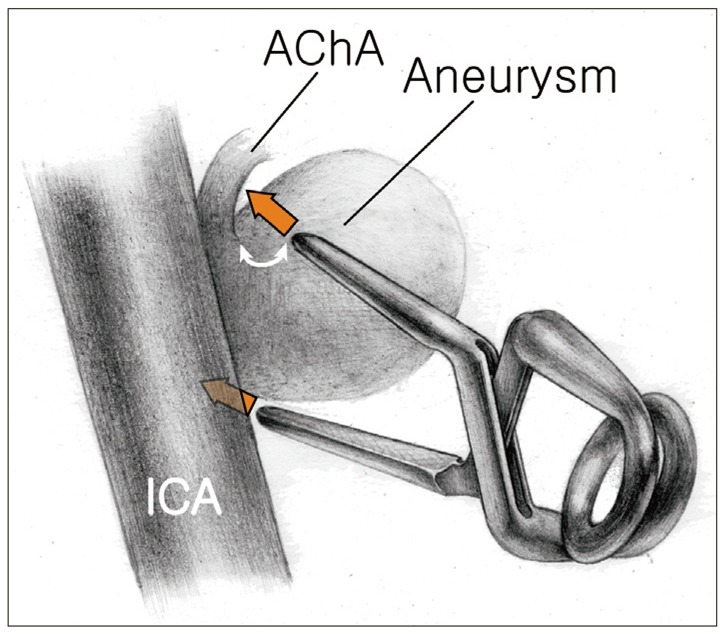
Illustration showing clip placement when an AChA arises inferior to aneurysm. The proximal blade of the clip is positioned a small distance away from the origin of the AChA so as not to constrict the AChA, while the distal blade is positioned adjacent to the wall of the ICA. Note the distance between the AChA origin and the clip blades (arrow). AChA: anterior choroidal artery, ICA: internal carotid artery.
Meanwhile, when the AChA arises posterior to the aneurysm base, the AChA origin is visualized by gentle compression of the aneurysm, while avoiding any inadvertent injury to the neck of the aneurysm. A straight clip is then applied across the neck of the aneurysm perpendicular to the axis of the ICA, being tilted laterally away from the origin of the AChA (Fig. 2). When manipulation of the aneurysm is limited by a thick wall or large size of the aneurysm, the use of an endoscope can be helpful. Furthermore, even when the AChA origin is not visible behind the aneurysm, its presence should still be assumed present until proven otherwise and the clip only placed in a safe area. Intraoperative monitoring, such as a motor evoked potentials (MEPs), is crucial in this situation.
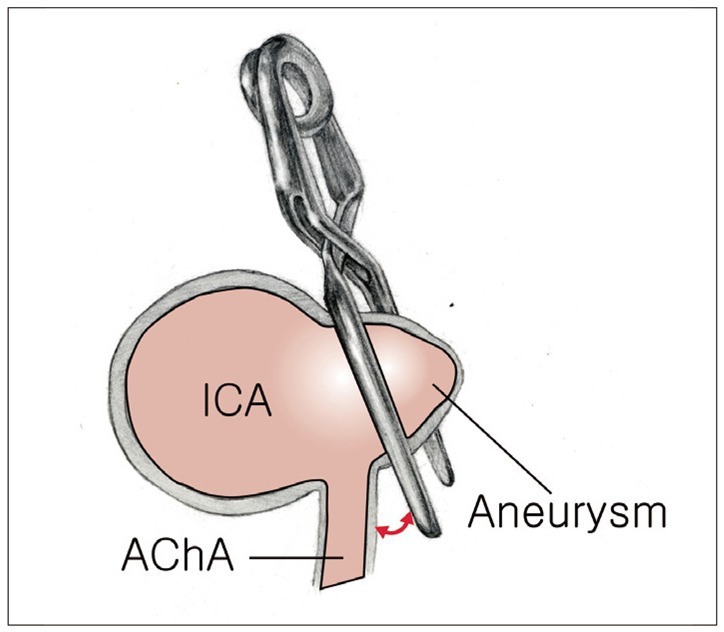
Illustration showing clip placement when AChA arises posterior to aneurysm base. A straight clip is applied across the base of the aneurysm perpendicular to the axis of the ICA, while tilting laterally away from the origin of the AChA. Note the distance between the AChA origin and the clip blades (red arrow). AChA: anterior choroidal artery, ICA: internal carotid artery.
After placing the clip, the clip blades are inspected to ensure complete occlusion of the aneurysm, and that the AChA is not compromised and the oculomotor nerve is free. Additional verification of the AChA blood flow can be obtained using microvascular Doppler sonography, indocyanine green near-infrared angiography, and MEPs2,3,7). If the dome of the aneurysm is adherent to the oculomotor nerve, it should be divided or punctured with microscissors rather than dissected from the nerve in order to avoid nerve injury.
Statistical analysis
The data in this report are presented as the mean±standard deviations. The statistical analyses were performed with the aid of an SPSS package (version 18 for Windows; SPSS, Inc., Chicago, IL, USA). A χ2-test was used to evaluate the affects of the preoperative World Federation of Neurologic Surgeons (WFNS) grade on the clinical outcomes (mRS). The results were considered significant for probability values less than 0.05.
RESULTS
Clinical and angiographic characteristics
The clinical and angiographic characteristics of the 62 patients with an AChA aneurysm are summarized in Table 1. The patient ages ranged from 32 to 75 years [mean±standard deviation (SD): 54.9±9.7 yr]. Thirty-six (58.1%) patients presented with an acute subarachnoid hemorrhage related to a ruptured AChA aneurysm (n=21) or other ruptured aneurysm (n=15), while 26 patients had an unruptured AChA aneurysm that was asymptomatic (n=24) or symptomatic (n=2) with oculomotor nerve palsy. Thirty-two (51.6%) patients presented with WFNS grade 1 to 3, while 4 (6.5%) patients presented with WFNS grade 4 to 5.
In the preoperative angiograms, the aneurysm height, i.e. the maximal perpendicular distance from the neck plane to the top of the aneurysm dome, ranged from 3 to 13 mm (mean±SD: 6.0±2.2 mm), while the maximum transverse diameter of the aneurysm neck ranged from 2 to 7 mm (mean±SD: 3.9±0.8 mm) and the maximum aneurysm diameter, which is the largest of all cross-sections along the height of the aneurysm, ranged from 3 to 12 mm (mean±SD: 4.5±1.4 mm).
The origins of the AChA in relation to the aneurysm were identified in the preoperative angiograms, and emitted from the base of the aneurysm in 30 (48.4%) patients and from the junction of the ICA and the aneurysm in 32 (51.6%) patients.
Surgery
Successful aneurysm clipping was achieved in all patients. For the patients (n=20) with a ruptured AChA aneurysm and who presented with WFNS grade 1 to 4, the operation was performed within a few hours after admission. However, for the patient who presented with WFNS grade 5, consent for surgery was only obtained when the patient's level of consciousness improved to Glasgow Coma Scale (GCS) score 7 (WFNS grade 4). The operation was then performed the day after admission.
A pterional approach was performed for most patients with a subarachnoid hemorrhage (pterional approach in 33 cases; supraorbital keyhole approach in 4 cases), whereas a supraorbital keyhole approach was applied for most of the unruptured cases (supraorbital keyhole approach in 18 cases; pterional approach in 7 cases).
Angiographic outcomes
All patients underwent postoperative angiographic evaluation using three-dimensional computed tomography angiography. Digital subtraction angiography was performed in 18 cases at the surgeon's discretion on the basis of surgical findings suggesting the possibility of incomplete aneurysm obliteration.
Fifty-seven (91.9%) patients exhibited complete aneurysm clipping, while five (8.1%) patients showed a small residual neck, where the incomplete clipping of the aneurysms was caused by intentional placement of the clip beyond the aneurysm neck to avoid AChA insufficiency. In these cases, an atherosclerotic aneurysm base, the AChA densely adherent to the aneurysm base, and complex aneurysm shapes led to the incomplete obliteration.
Surgical complications
In the present series, there was no case of permanent AChA syndrome or oculomotor nerve palsy induced by the surgical procedures. However, three (4.8%) patients suffered transient AChA syndrome lasting less than a week postoperatively.
For a 63-year-old woman harboring a small unruptured AChA aneurysm with a daughter sac, the AChA aneurysm was exposed via a supraorbital keyhole approach. The location of the AChA origin behind the aneurysm was ascertained by gentle compression of the aneurysm. A side-curved clip was then placed nuzzled close to the origin of the AChA, yet leading to inadvertent constriction of the AChA (Fig. 3A-D). However, this was not discovered as MEPs were not routinely determined in the early cases of the present series and the Microvascular Doppler was out of order.

Complicated case with postoperative hemiplegia. A: Intraoperative photograph showing AChA aneurysm with daughter sac. B: Intraoperative photograph showing AChA origin posterior to aneurysm base, reveals when compressing the aneurysm with blunt dissecter. C: Intraoperative photograph showing initial clip placement using side-curved clip. D: Illustration showing initial clip placement and resultant constriction of AChA origin. E: Intraoperative photograph showing changed clip placement using straight clip tilted laterally in immediate reoperation. F: Illustration showing changed clip placement to obtain luminal patency of AChA. AChA: anterior choroidal artery, ICA: internal carotid artery.
When the patient awoke from surgery in the operating room, she exhibited contralateral hemiplegia, so a re-operation was performed immediately without radiological verification of an AChA occlusion and the curved clip replaced with a straight one placed further away from the AChA origin (Fig. 3E, F). The duration of the AChA occlusion, from the initial clip placement to the clip repositioning, was only 70 minutes. The patient then awoke with an improved hemiparesis (grade 3/5), and recovered completely after 6 hours.
Clinical outcomes
The clinical outcomes of the patients in this series were found to be related to their preoperative WFNS grade (Fig. 4). As regards the 3-month mRS, significant differences were found between the patients with an unruptured aneurysm (WFNS grade 0; n=26), good-grade subarachnoid hemorrhage (WFNS grade 1 to 3; n=32), and poor-grade subarachnoid hemorrhage (WFNS grade 4; n=4) (p=0.000).

Bar graph showing significant influence of WFNS grade on 3-month mRS in patients with AChA aneurysm. AChA: anterior choroidal artery, ICA: internal carotid artery, mRS: modified Rankin Scale, WFNS: World Federation of Neurologic Surgeons.
The patients (n=26) without an acute subarachnoid hemorrhage remained neurologically intact or improved from their preoperative clinical status. Two patients with an AChA aneurysm inducing oculomotor nerve palsy recovered completely after 3 months, while the other 24 patients with an asymptomatic unruptured aneurysm remained neurologically intact postoperatively.
Among the patients (n=36) with an acute subarachnoid hemorrhage, 32 were WFNS grade 1 to 3, while 4 were grade 4 to 5. For the WFNS grade 1 to 3 patients, the 3-month mRS score was 0 (n=26, 81.3%) or 1 (n=6, 18.8%). Meanwhile, for the 4 WFNS grade 4 to 5 patients, the 3-month mRS score was 1 (n=1, 25.0%) or 2 (n=3, 75.0%), where their symptoms and disabilities were related to a cognitive dysfunction.
For the patients who presented with a subarachnoid hemorrhage, the AChA aneurysm was ruptured in 21 patients, while other anterior circulation aneurysms arising at the PCoA origin (n=7), ICA bifurcation (n=1), anterior communicating artery (n=5), and middle cerebral artery (n=2) were ruptured in 15 patients. Four (19.0%) of the 21 patients with a ruptured AChA aneurysm presented with contralateral hemiparesis or hemiplegia on admission (Fig. 5). The CT scans of 3 hemiplegic patients revealed a caudoputaminal hemorrhage, massive intraventricular hemorrhage, and considerable amount of cisternal bleeding on the insular cortex, respectively, but no direct disruption of the corticospinal tract was observed. Their preoperative GCS score was 7 to 12. The clinical outcomes were favorable with 3-month mRS scores of 1 or 2 and complete recovery from hemiplegia within 6 months.

Clinical characteristics and imaging results for 4 patients who presented with contralateral hemiparesis after AChA aneurysm rupture on admission. AChA: anterior choroidal artery, GCS: Glasgow Coma Scale, mRS: modified Rankin Scale, WFNS: World Federation of Neurologic Surgeons.
Oculomotor nerve palsy in relation to ruptured (n=2) and unruptured (n=2) AChA aneurysms was observed in four patients on admission. However, total recovery from the complete (n=2) and incomplete (n=2) palsy was experienced within 3 months.
No recurrence or rebleeding of the clipped aneurysms occurred during the follow-up period, ranging from 3 months to 7 years (mean±SD: 3.2±2.2 years).
DISCUSSION
Due to the low incidence of AChA aneurysms, only 2 to 5% of intracranial aneurysms4,19), there are relatively few reports on the related outcomes of surgical and endovascular treatments, as summarized in Table 2 and 31,5,6,8-11,15-17,20), which only include studies of more than 10 individuals with a non-giant aneurysm published in a peer-reviewed journal.
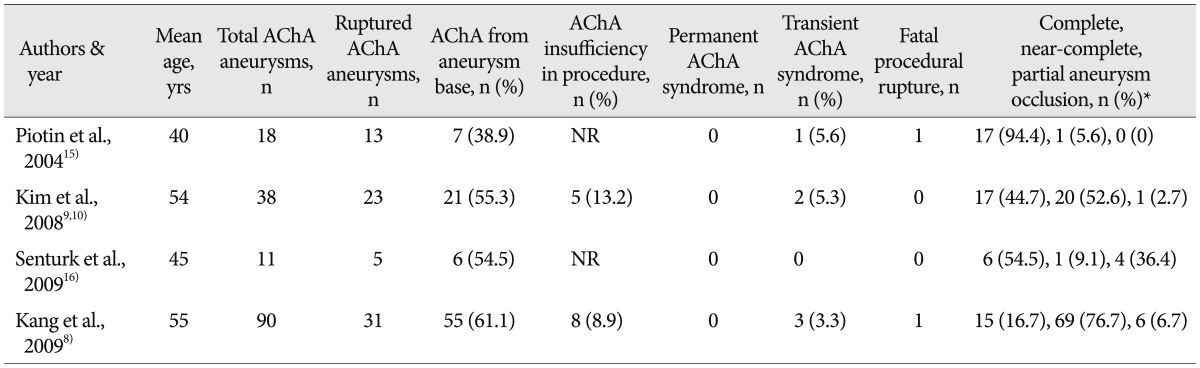
Literature review of procedural complications after endovascular treatment of non-giant anterior choroidal artery aneurysms
AChA insufficiency as an ischemic complication of aneurysm clipping develops into transient or permanent AChA syndrome, which is typified by contralateral hemiplegia/hemiparesis with less consistent contralateral hemianesthesia or hemianopsia. The incidence of permanent AChA syndrome after surgical clipping of an AChA aneurysm has consistently been reported to range from 5.3% to 15.7%1,5,6,10,11,17,20). When compared with surgical series, endovascular series have a much lower incidence of AChA insufficiency, although only a few series have been reported8-10,15,16). However, the favorable surgical outcomes of the current series, no occurrence of permanent AChA syndrome in the 62 patients, would seem to suggest that the risk of AChA insufficiency can be reduced with appropriate surgical techniques and intraoperative monitoring18).
While the surgical access to an AChA aneurysm after dural opening in a craniotomy is technically simple and straightforward, the surgical results are actually determined by the ensuing maneuvers, including identification of the AChA at its origin and around the aneurysm, careful microdissection separating the AChA from the aneurysm, and appropriate clip application. Preserving the patency of the AChA is sometimes demanding and requires careful attention to avoid a devastating postoperative AChA infarction.
Regarding the endovascular coiling of AChA aneurysms, the previous reports show that endovascular treatment is effective for preventing aneurysmal ruptures and recurrent bleeding with low morbidity and mortality rates8-10,15,16). However, there are significant concerns with an endovascular procedure, including thrombotic occlusion of the AChA, a fatal procedural aneurysm rupture, and high recurrence rate of occluded aneurysms over a long-term period8-10,14-16).
The case of an AChA aneurysm where the AChA arises from the aneurysm base is a more challenging situation to preserve the AChA patency for both surgical and endovascular treatment. In the current and six other series that reported on the origin of the AChA in association with the aneurysm (Table 2, 3), a total of 172 (45.5%) out of 378 aneurysms had the AChA arising from the aneurysm base rather than the junction of the ICA and the aneurysm1,8,9,11,15,16).
In Yasargil's report on 21 patients with a ruptured AChA aneurysm19), four patients presented with contralateral hemiparesis on admission. These patients showed an incomplete recovery of hemiparesis (n=2), aggravated hemiparesis due to AChA occlusion after aneurysm clipping (n=1), and early death related to severe brain swelling and a large hemispheric infarct (n=1). The reasons for the 2 cases of incomplete hemiparesis recovery, in contrast to the 4 cases of complete recovery of initial hemiparesis in the present series, cannot be explained as no CT scans were available at that time.
Although the current study is limited based on a retrospective review of a case series from a single institution, it may help reappraise the surgical outcomes of AChA aneurysms and contribute to the limited available literature.
CONCLUSION
Surgical treatment of AChA aneurysms involves a significant risk of AChA insufficiency, yet this risk can be minimized by careful effort to preserve the AChA patency based on AChA-avoiding clip placement and intraoperative monitoring. In addition, the surgical outcome is primarily determined by the preoperative clinical state (WFNS grade).
Acknowledgements
This study was supported by a grant from the Korea Healthcare Technology R & D Project, Ministry of Health & Welfare (A100870), Republic of Korea.

