Usefulness of Dexmedetomidine during Intracerebral Aneurysm Coiling
Article information
Abstract
Objective
General anesthesia is often preferred for endovascular coiling of intracranial aneurysm at most centers. But in the authors' hospital, it is performed under monitored anesthesia care (MAC) using dexmedetomidine. To determine the feasibility and safety of this approach, the authors reviewed our initial experience.
Methods
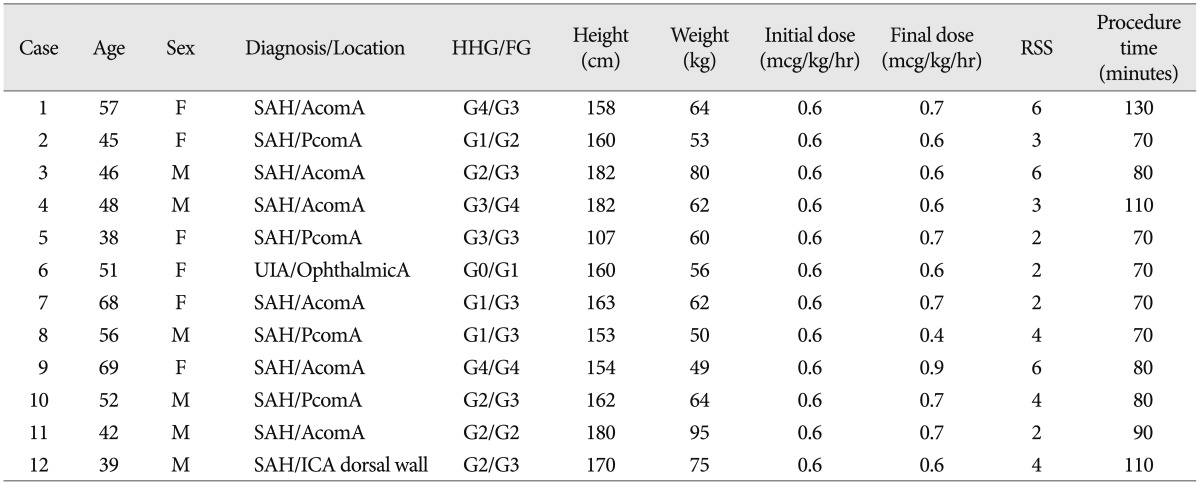
Retrospective data was analyzed from July 2012 to November 2012. We performed coil embolization in 28 cases using this method. Among them, for statistical significance, we analyzed 12 cases in which the procedure time exceeded an hour. Vital signs were analyzed every 10 minutes. Depth of sedation was measured according to the Ramsay sedation scale and frequency of the repeated roadmap image(s) caused by movement of the patient's head during the procedure.
Results
All procedures were completed without occurrence of procedure related complications. Under MAC using dexmedetomidine, vital signs of the patients were stable, no statistical significance regarding hemodynamic and respiratory parameters was observed between time points (p>0.05). Adequate sedation was achieved. Mean Ramsay sedation scale was 3.67±1.61 (2 to 6). Repeated roadmap image(s) due to patient's factor occurred in only one case. The mean dosage of drug for adequate sedation for the procedure was 0.65±0.12 mcg/kg/hr without loading doses.
Conclusion
To the best of my knowledge, this is the first report published in English using the method of monitored anesthesia with dexmedetomidine for intracranial aneurysm coiling. Monitored anesthesia care using dexmedetomidine without loading dose for embolization of intracranial aneurysms appeared to be a safe and effective alternative to general anesthesia.
INTRODUCTION
Coil embolization has recently emerged as a treatment modality for intracranial aneurysms. Advances in materials, devices, and technique for endovascular procedures have allowed for emergence of the endovascular approach for intracranial aneurysms as a safe and often effective alternative to microsurgical clipping.
In endovascular treatment, the general goals of anesthetic management involve hemodynamic control in order to minimize the risk of aneurysm rebleeding and strategies to protect the brain from ischemic injury5). There is limited information in the literature pertaining to anesthetic management of patients undergoing endovascular treatment of intracranial aneurysms7,19). In general, the anesthetic principles that apply to transcranial microsurgical treatment for cerebral aneurysms also apply to endovascular treatment. However, the coils are delivered using an endovascular approach that does not involve extensive surgical excision, therefore, administration of general anesthesia is not mandatory. In addition, general anesthesia is not always available for all neurointervention. Therefore, the choice of anesthetic technique varies depending on the status of each institution; the most common techniques are conscious sedation and general anesthesia11,14). Another goal of the anesthesia is keeping the patient motionless in order to optimize the quality of the images used in performance of the endovascular procedure: hence, general anesthesia with endotracheal intubation is often preferred for these procedures5). However, general anesthesia does not allow for intraprocedural evaluation of the patient's neurological status and carries additional risks associated with general anesthesia and mechanical ventilation14). Coil embolization of the intracranial aneurysm can be performed under monitored anesthesia care (MAC) with dexmedetomidine, however, the safety and feasibility of this procedures has not been studied yet. At the authors' institute, coil embolization of the intracranial aneurysm is performed under MAC with dexmedetomidine. In order to determine the feasibility and safety of this approach, the author has reviewed our initial clinical experience using MAC with dexmedetomidine for aneurysmal coiling.
MATERIALS AND METHODS
Patient population and data collection
Retrospective analysis of prospectively collected data in a single institution was conducted during the period from July 2012 to November 2012. During this period, endovascular procedure of the intracranial aneurysms was performed in 28 cases under MAC with dexmedetomidine. Sixteen cases with procedure time of less than 60 minutes were excluded for the following reason. Onset time of dexmedetomidine at intravenous infusion is 5-10 minutes and the peak effect will appear 15-30 minutes after startup. And, for analysis of the impact of the drug on patients by repeated measures analysis of variance (repeated measures ANOVA), more than three times repetitive measurements are required. In addition, the patients' vital signs were checked every 10 minutes during the procedures. Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (mABP), heart rate (HR), respiratory rate (RR), and peripheral oxygen saturation (SpO2) were recorded at each time point, as follows: preoperative baseline, at anesthesia start, after every 10 minutes until the end of the procedures. Thus, we included 12 cases with a procedure time of more than one hour for statistical analysis. Of 12 cases, one is unruptured intracranial aneurysm case and the others are subarachnoid hemorrhage cases.
Another goal of the anesthesia is keeping patients motionless during the procedures. Adequate sedation was measured using two methods. The first one was the frequency of the repeated road-map image(s) caused by the patients' factor. Frequent roadmap means unnecessary motion of the patients. The second one was Ramsay sedation scale (RSS). It was used for assessment of the level of sedation of the patients (Table 1)16).
RESULTS
Coil embolization was performed under MAC with dexmedetomidine in 12 cases according to the MAC protocol (Table 2). The procedure was completed successfully without occurrence of any complication in all cases (100%). A summary of the clinical characteristics of the patients is shown in Table 3. Adequate sedation for the endovascular procedure was achieved in all cases. Mean RSS was 3.67±1.61 (RSS 2 to 6). Repeated roadmap image due to patient's factor occurred in only one case. The mean dosage of drug for adequate sedation was 0.65±0.12 mcg/kg/hr (0.4-0.9 mcg/kg/hr) without loading doses. All patients returned to their previous state, prior to the drug injection, within a few minutes to stop the drug infusion.
Changes of hemodynamic and respiratory variables are shown in Fig. 1,2,3. All vital signs (SBP, DBP, mABP, HH, RR, and SpO2) of the patients also did not change throughout the procedures. No statistically significant change in hemodynamic and respiratory parameters was observed between time points (p value>0.05) (Table 4).

Change of hemodynamic variable (SBP, DBP) according to procedure time. Systolic and diastolic blood pressure of the patients did not change during periods of dexmedetomidine infusion and it did not show statistical significance (p>0.05). SBP : systolic blood pressure, DBP : diastolic blood pressure.
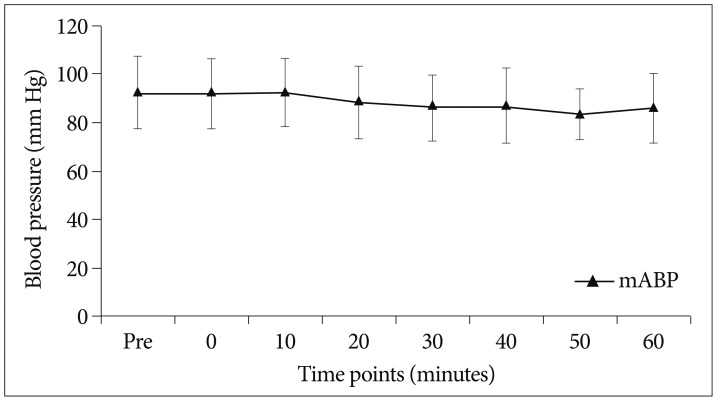
Change of hemodynamic variable (mABP) according procedure time. The mABP of the patients did not change during periods of dexmedetomidine infusion and it also did not show statistical significance (p>0.05). mABP : mean arterial blood pressure.
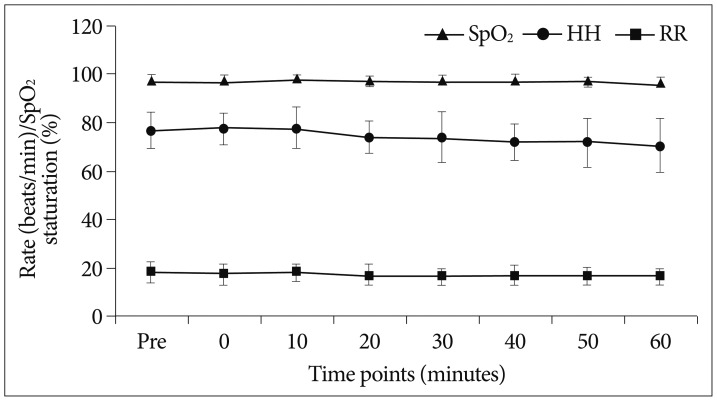
Change of respiratory variables (HR, RR, SpO2) according to procedure time. HR, RR, and SpO2 of the patients did not change during periods of dexmedetomidine infusion and it did not show statistical significance (p>0.05). HR : heart rate, RR : respiratory rate, SpO2 : peripheral oxygen saturation.
DISCUSSION
Anxiolysis and sedation
The mean RSS of this report was 3.67±1.61 (range, 2-6). RSS score of 3 points indicates moderate sedation, conscious sedation with anxiolysis17). It means that the patients purposeful response to verbal or tactile stimulation but no feeling of anxiety. In other words, the patients will remain motionless without external auditory or tactile stimuli. We think that this is the most appropriate situation for endovascular procedures. In this situation, the patient is stable and there is no fear or pain, the physician can also perform the endovascular procedure comfortably without movement of the patients. This state permits regular neurological evaluations throughout the procedure with a light stimulation. Deeper sedation in RSS seems to be relatively bad. In one case, we had to be repetitive roadmaps in deep sedation state. Deep sedation means that the patient showed a response after repeated or painful stimulation17). The patient fell quickly into a deep sleep, and began to snore. However, periodical subtle head movement with snoring has a negative influence on endovascular procedures.
Light sleeping without motion during the procedure is ideal. Thus maintenance of proper drug level and adequate depth of sedation are the most important considering factor for safe endovascular procedure. And adequate depth of sedation can be achieved easily with adjustment of infusion rate of the drug within 1-2 minutes.
Dexmedetomidine is known as a more specific and selective α2-adrenergic receptor agonist with a shorter elimination half-life. Dexmedetomidine appears to exert its sedative and anxiolytic effects through activation of α2-adrenoreceptors in the locus ceruleus, a major site of noradrenergic innervation in the central nervous system (CNS). The sedation produced by α2-adrenoreceptor agonists, unlike that produced by traditional sedatives, such as benzodiazepines and propofol, does not depend primarily on activation of the α-aminobutyric acid (GABA) system. In addition, the primary site of α2-agonist sedative action does not appear to be the cerebral cortex, as would be the case eiwith GABA-mimetic drugs3). Perhaps because of a noncortical site of action, α2-agonists appear to engender a different type of sedation compared with GABA-mimetic drugs. Dexmedetomidine produces an unusually cooperative form of sedation, in which patients easily transit from sleep to wakefulness and task performance when aroused, and then back to sleep when not stimulated12,21). As it acts in a different way and the half-time is very short, it is very easy to regulate the appropriate drug concentration depending on the patient's depth of sedation.
Analgesia
Severe bursting headache is a unique symptom of the aneurysmal subarachnoid hemorrhage. Analgesic such as acetaminophen or morphine is routinely necessary for relief of the thunderclap headache. The intensity of headache was not measured in this study, but there were no patients' head movement due to intolerable headache without other analgesics. The α2-Agonists have been recognized as having significant analgesic effects. Dexmedetomidine has been shown to consistently reduce opioid requirements by 30 to 50%1,20). The precise mechanisms and pathways by which drugs in this class induce analgesia have not been fully elucidated. Brain, spinal cord, and peripheral mechanisms all appear to be operant.
Cardiovascular effects
Our study shows hemodynamic stability. No changes in vital signs (SBP, DBP, mABP, HH) of the patients were observed throughout the procedures. Vital signs of the patients showed a decreasing tendency, however, no statistical significance was observed between time points (p>0.05). The decreasing tendency is not the drug effect but normal physiological change perhaps coupled with relief of pain, reduced anxiety, and prolonged rest. The hemodynamic effects of dexmedetomidine resulted from peripheral and central mechanisms. The α2B-receptors located on vascular smooth muscle mediate vasoconstriction. The initial response to rapid dexmedetomidine infusion may be a transient hypertension4,9). In the CNS, activation of α2-receptors leads to a reduction in sympathetic outflow and an increase in vagal activity. In addition, dexmedetomidine may have some action as a peripheral ganglionic blocker, further enhancing the sympatholytic effect13). The net effect of α2-adrenoreceptor action is a significant reduction in circulating catecholamines, modest reduction in blood pressure, and modest reduction in heart rate10,18). Although hypotension has been described in patients receiving dexmedetomidine, this exaggerated physiological effect appears to have a frequent temporal relationship with the use of a loading dose and/or preexisting hypovolemia8). If we use maintaining dose of drug without loading dose, we can perform coil embolization without hypotension and bradycardia. Lee et al.10) also reported on linear pharmacokinetics characteristics and dose-dependent sedation effects in Korean subjects. With loading and maintenance dose of the drugs, sedative effects were significantly increased by dexmedetomidine compared to placebo. Blood pressure and heart rate were decreased, however, stable oxygen saturation was maintained.
Effects on ventilation
Our reports showed that stable respiratory function and peripheral oxygen saturation were maintained (over 94%) during the procedure. The α2-adrenoreceptor agonists have minimal effects on ventilation4,12). In healthy volunteers, as well as intraoperatively, even very high doses of dexmedetomidine did not compromise respiratory function10). Respiratory rates actually showed an upward trend throughout the dose escalation, which eventually reached 14 times the therapeutic plasma level6). The lack of respiratory depression, as measured by pulse oximetry and PaCO2, was also demonstrated in patients sedated with dexmedetomidine, which was administered at infusion rates 10 to 15 times higher than maximally recommended15).
Effect of intracranial pressure
In previous animal studies on rabbits22). intracranial pressure (ICP) either decreased or did not change with the administration of dexmedetomidine. In the other study2), there was slight decrease in the mean for ICP and a slight corresponding increase in cerebral perfusion pressure (CPP). But the standard deviation between ICP and CPP was small, having no clinically relevant deleterious change2). This shows that dexmedetomidine could be administered safely without clinically affecting either ICP or CPP2).
CONCLUSION
Without loading dose of dexmedetomidine, the authors were able to achieve adequate sedation and sufficient pain control during the endovascular coiling without hemodynamic and respiratory depression. Monitored anesthesia care using dexmedetomidine for embolization of intracranial aneurysms appeared to be a safe and effective alternative to general anesthesia.
Acknowledgements
This study was supported by research fund from Yeungnam University, 2011.
The work has been accepted for 10th SNFS Annual Meeting 2013.