Intramedullary Solitary Fibrous Tumor of Cervicothoracic Spinal Cord
Article information
Abstract
Solitary fibrous tumor is rare benign mesenchymal neoplasm. The spinal solitary fibrous tumor is extremely rare. The authors experienced a case of intramedullary solitary fibrous tumor of cervicothoracic spinal cord in a 48-year-old man with right lower extremity sensory disturbance. Spinal MRI showed intradural mass lesion in the level of C7-T1, the margin between the spinal cord and tumor was not clear on MRI. A Left unilateral laminectomy and mass removal was performed. Intra operative finding, the tumor boundary was unclear from spinal cord and it had intramedullary and extramedullary portion. After surgery, patient had good recovery and had uneventful prognosis. Follow up spinal MRI showed no recurrence of tumor.
INTRODUCTION
Solitary fibrous tumor is rare lesion that arises commonly in the visceral pleura, submesothelial connective tissue4,14). Carneiro et al.6), reported the first two cases of spinal solitary fibrous tumor in 1996. Nowadays, wider understanding of this type of tumor has led to the discovery of its occurrence in various extrathoracic and nonserosal site. From Carneiro's first report, through our literature review, only 30 cases have been reported. So far, we report a new case of a patient with intramedullary solitary fibrous tumor occurring in the cervicothoracic spinal cord.
CASE REPORT
A 48-year-old man was admitted to our hospital in due to right lower extremity sensory disturbance which was started one month prior to admission. On general appearance, this patient looked healthy. However, his neurological examination revealed right sided parestheisa and hyposthesia to touch sensation below T5 dermatome. Right knee and ankle jerk were hypoactive. Babinski sign was present bilaterally. There was no motor weakness.
A magnetic resonance imaging (MRI) revealed an intradural extramedullary (IDEM) mass that was an isointense lesion on T1-WI and a slightly hyperintense on T2-WI, lying dorsal and left lateral to the spinal cord in the level of C7-T1. Spinal IDEM mass showed an intense enhancement on MRI after gadolinium administration (Fig. 1). Since the margin between the spinal cord and tumor was not clear on MRI, we preoperatively, could not get confidence about location of tumor, whether the tumor was intramedullary or extramedullary.
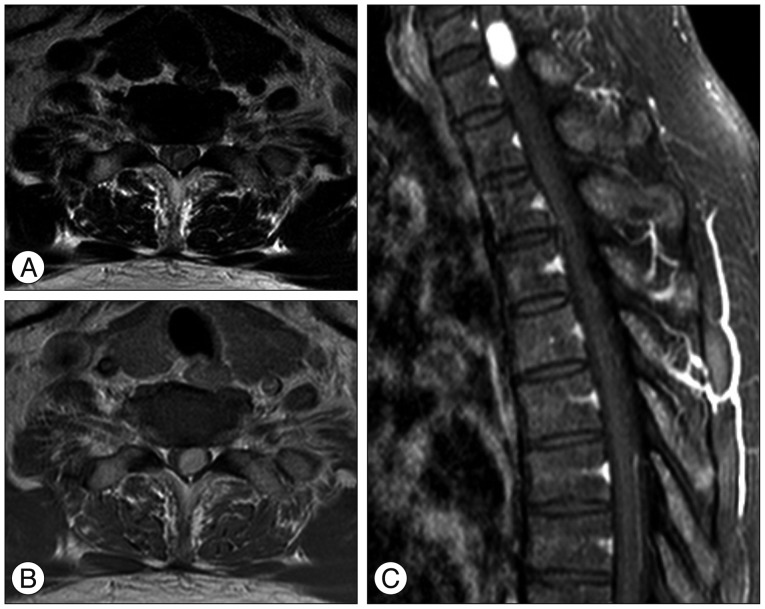
Preoperative axial (A and B) sagittal (C) MRIs demonstrating a left-sided intra-dural spinal cord tumor at the level of C7-T1. The tumor is homogenously enhanced after gadolinium injection. The tumor margin is unclear to spinal cord.
The operation was performed in the prone position. Intra-operative neuromonitoring was done. A rigid head holder was used to secure the head in a neutral position. A left unilateral laminectomy was performed with drill and Kerrison punch at the C7-T1 level. The exposed dura mater was found to be tensed. With the aid of microscope, the dura was then opened straightly in the midline. A hypervascular white-yellowish hard tumor was found. The tumor was located the left posterolateral surface of spinal cord compressing and shifting and shifted to the right of the spinal canal (Fig. 2). The internal debulking was done. The intra-operative examination of the frozen sections revealed in a preliminary diagnosis of a benign tumor with a suspicion of neurogenic tumor with hypercellularity. The tumor boundary was unclear from spinal cord and it had intramedullary and extramedullary portion. The internal debulking was performed as much as possible to decompress the cord. After resection and hemostasis, the dura was then closed primarily in a watertight fashion. The histopathological diagnosis was spindle cell tumor without necrosis and mitosis, immunostaining was positive only for CD34, while S-100 protein, epithelial membrane Ag (EMA) were negative (Fig. 2).
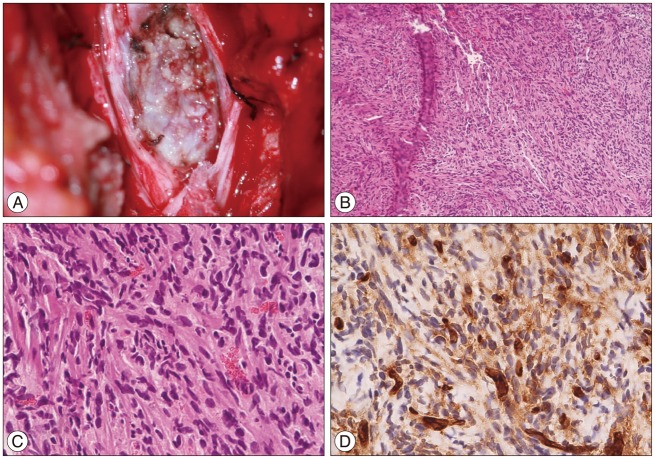
Intraoperative photograph (A) showing the tumor strongly adherent to pia mater and arising within the spinal cord, without clear margin. Photomicrographs of the specimen showing a proliferation of predominantly spindle-shape cells and intracellular collagen deposition (B : HE stain, ×40, C : HE stain ×400), and immune-histochemical staining revealing strong, diffuse positive for CD34 (D).
Initial follow-up MR image was done at 2 weeks after surgery and showed remnant tumor. There is, however, no evidence of tumor recurrence on further MRI scan at 6 months after operation (Fig. 3). The patient had good recovery after operation and had uneventful prognosis without any sign and symptoms suggesting recurrence of tumor.
DISCUSSION
Solitary fibrous tumor is rare benign mesenchymal neoplasm and has been reported arising in soft tissues elsewhere. The spinal solitary fibrous tumor is extremely rare. This case report, hence adds one additional case of reported spinal solitary fibrous tumor. Clinically, the patient was middle-aged man without any medical history who sought medical care for neurologic symptoms caused by medullary compression.
We found 30 reported cases of spinal fibrous tumor in various published literature. Muñoz et al.16) reported that mean age at diagnosis was 46.5; male-to-female ratio was 1.4 : 1; Predominantly affected location was thoracic spine, followed by cervical spine, and most produced symptoms consisted of pain, myelopathy, radiculopathy, and motor or sensory deficit. Through our literature review, mean age at diagnosis was 46; male n=16, female n=14; predominantly affected location was thoracic spine (53.3%), followed by cervical spine (33.4%), lumbar spine (6.7%) and sacrum (3.3%). Spinal solitary fibrous tumor are mostly intramedullary (13) or intradural and extramedullary (12). Only five cases of extradural spinal fibrous tumors have been reported so far. In current case, the patient's age (5th decade) and location of tumor at intramedullary cord of cervicothoracic junction, this is typical case of spinal solitary fibrous tumor.
Solitary fibrous tumor must be differentiated from fibrous meningioma, hemangiopericytoma. The distinct microscopic findings of solitary fibrous tumor are dense collagenous band and lacks of specific structural feature, such as well-formed lobules, whorls, or psammoma bodies8,12). Immunohistochemical stains are positive for CD34 antigen, whereas meningiomas are positive for EMA and schwannomas for S-1006,17). Immunoreactivity of the tumor to CD34 and negative to S-100 and EMA distinguish solitary fibrous tumors from the other spindle cell tumor. However, hemangiopeircytoma may be positive for CD34 antigen, but these tumors are typically hypercellular with mitosis and necrosis12). The solitary fibrous tumor usually exhibit strong, diffuse reactivity for CD34 whereas hemangiopericytoma show pachy, focal expression of CD348).
Solitary fibrous tumors are usually indolent neoplasm that may be cured by complete surgical resection. However, thoracic solitary fibrous tumor with malignant transformation has been reported in 10 to 15%20). Histological benign solitary fibrous tumor may also be transformed to malignancy even after several years of diagnosis9). In such cases, metastasis and recurrence may take place even after total resection6,15). Through our literature review, we found two cases of malignant spinal solitary fibrous tumor (6.7%)3,16) and three cases of recurred tumor (10%)2,6,11). Malignant solitary fibrous tumors usually include high cellularity, cellular pleomorphism, necrosis, and high mitotic count (mitotic index >4 mitoses per 10 HPF)16,19,20). Expression of Ki-67 may be helpful to determine the possibility of malignant transformation. Muñoz et al.16) reported a case of malignant spinal solitary fibrous tumor. The tumor had a low mitotic count (<5/50 HPF), without the presence of pleomorphism or necrosis and exhibited strong and diffuse positive for CD34 and vimentin with low expression of Ki-67 (-1%). As the tumor have transformed to malignancy which diminished CD34 expression and high expression of Ki-67 (up to 10%). Diminished CD34, positive p53 immunoreactivity, and high expression of Ki-67 have been related to secondary malignant transformation of solitary fibrous tumors5,7,21). For our case, we need to follow up the patient for long time to detect the malignant change or recurrence of tumor.
The curative treatment of solitary fibrous tumor is complete surgical excision18). This strategy was supported by rationale that the recurred tumors had poorer prognosis compared to primary tumors. Moreover, recurrence after gross total removal of solitary fibrous tumor is uncommon. But we are aware of three cases of local recurrences2,6,11). These cases were related to incomplete tumor resection because of large size, serious loss of blood, and inability of extensive radical resection. In our case, it was impossible to complete surgical resection due to severe adherence to pia mater and unclear tumor border along spinal cord. Adjuvant therapies, including chemotherapy or radiation, have been suggested if the solitary fibrous tumor resection is incomplete10). Radiation therapy for inoperative malignant solitary fibrous tumor had significant therapeutic response13). Hashimoto et al.11) presumed that palliative radiation therapy was effective in slowing down the tumor invasion. However, the study mentioned that the effectiveness of radiation therapy for solitary fibrous tumor needs further clinical studies on large number of patients. In other report2), recurred solitary fibrous tumor after partial excision was removed completely by two additional surgery. Adjuvant chemotherapy, ifosfamide and doxorubicin, for malignant spinal solitary fibrous tumor have been reported in two cases3,16). However, these systemic therapies have not yet been proven to be effective. For our case, we did not perform adjuvant therapy, because the histopathological finding of tumor was benign and follow up MRI did not show recurrence of tumor.
In conclusion, the spinal solitary fibrous tumor is extremely rare neoplasm among spinal cord lesions. These tumors usually have stationary growth rate but cause neurological symptom early due to compression of the spinal cord. Unfortunately, investigations, including MRI studies, do not differentiate from other primary intraspinal tumors1). Hence, in clinical setting, when we meet intraspinal tumor, we must consider possibility of solitary fibrous tumor and perform careful surgical planning for meticulous total resection.
