Cerebral Arteriovenous Malformation Associated with Moyamoya Disease
Article information
Abstract
The coexistence of moyamoya disease (MMD) with an arteriovenous malformation (AVM) is exceedingly rare. We report two cases of AVM associated with MMD. The first case was an incidental AVM diagnosed simultaneously with MMD. This AVM was managed expectantly after encephalo-duro-arterio-synangiosis (EDAS) as the main feeders stemmed from the internal carotid artery, which we believed would be obliterated with the progression of MMD. However, the AVM persisted with replacement of the internal carotid artery feeders by new external carotid artery feeders from the EDAS site. The AVM was eventually treated with gamma knife radiosurgery considering an increasing steal effect. The second case was a de novo AVM case. The patient was initially diagnosed with MMD, and acquired an AVM eight years later that was slowly fed by the reconstituted anterior cerebral artery. Because the patient remained asymptomatic, the AVM is currently being closely followed for more than 2 years without further surgical intervention. Possible differences in the pathogenesis and the radiologic presentation of these AVMs are discussed with a literature review. No solid consensus exists on the optimal treatment of MMD-associated AVMs. Gamma knife radiosurgery appears to be an effective treatment option for an incidental AVM. However, a de novo AVM may be managed expectantly considering the possible risks of damaging established collaterals, low flow characteristics, and probably low risks of rupture.
INTRODUCTION
Moyamoya disease (MMD) is a chronic cerebral vasculopathy characterized by progressive bilateral steno-occlusion at the terminal portion of the internal carotid artery (ICA), and/or at the proximal portion of the anterior cerebral artery (ACA), and/or the middle cerebral artery (MCA), with concomitant abnormal vascular networks in the vicinity of the steno-occlusive lesions4). The coexistence of MMD with an arteriovenous malformation (AVM) is very rare. We report two cases of MMD associated with AVM.
CASE REPORT
Case 1
A 12-year-old girl presented with a history of transient ischemic attacks (TIAs) on the right side for 2 years. MRI and MRA showed a signal void structure in the right parietal lobe and steno-occlusive vascular lesions mainly involving the left ICA and the left posterior cerebral artery. Neither infarction nor hemorrhage was detected in both cerebral hemispheres. A neurological examination showed no deficits on admission. The patient underwent cerebral angiography and was diagnosed with MMD by bilateral steno-occlusive lesions and the typical occurrence of abnormal vascular networks (Fig. 1A). Additionally, a small AVM was noted in the right parietal lobe supplied by the posterior parietal and angular arteries. Single photon emission computed tomography (SPECT) revealed global hypoperfusion in the left hemisphere and mild hypoperfusion in the right anterior temporal lobe. Encephalo-duro-arterio-synangiosis (EDAS) was performed on the left hemisphere using branches of the external carotid artery (ECA), followed by subsequent EDAS on the right hemisphere.

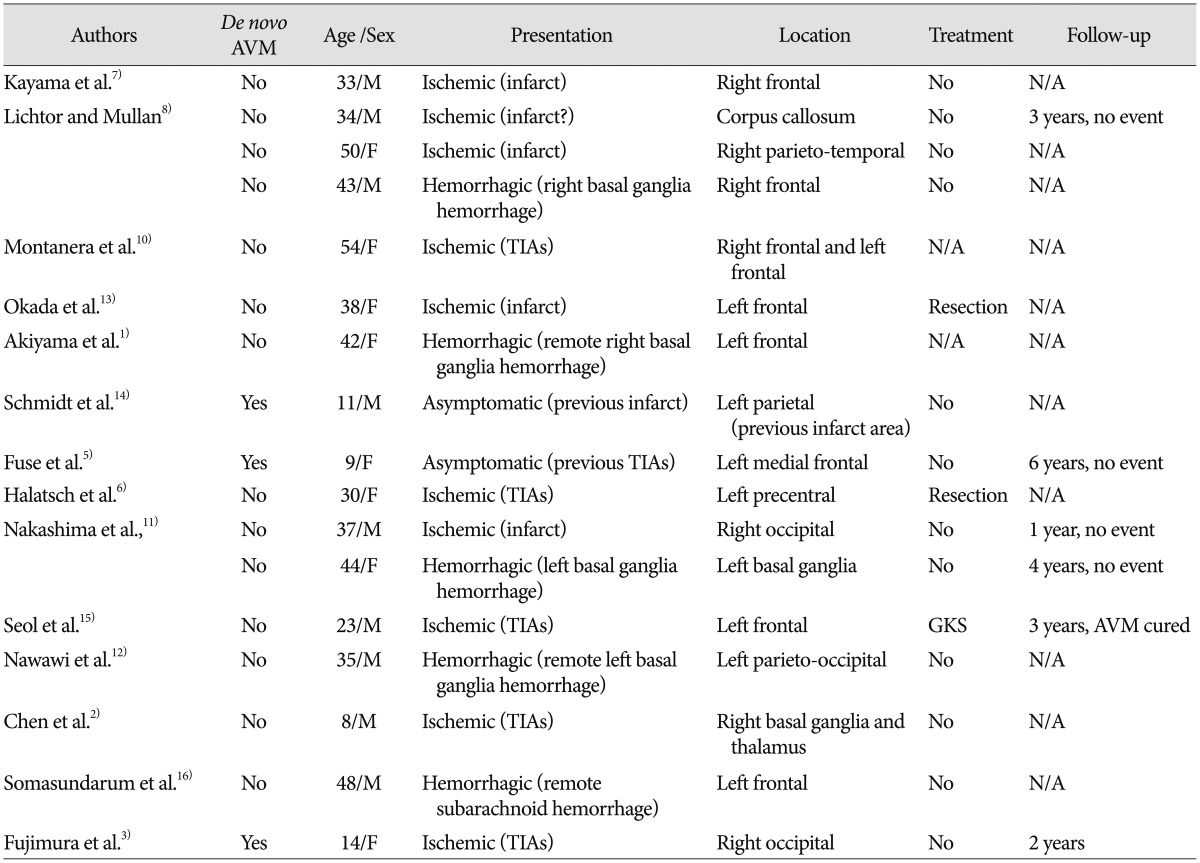
Case 1 images. A : Initial right ICA angiograms obtained in the late arterial phase, showing a small AVM in the right parietal lobe. Left ICA angiogram shows abnormal vascular networks suggestive of MMD. B : Follow-up angiography performed 4.5 years after EDAS, showing progressive stenosis of the right ICA with decreased flow to the AVM. Right ECA angiogram shows surgically established collaterals at the EDAS site. C : Subsequent angiography performed 9.5 years after EDAS, demonstrating newly-developed AVM feeders from the EDAS site. D : Obliteration of the AVM is strongly indicated by MRI taken 2 years after gamma knife radiosurgery. ICA : internal carotid artery, AVM : arteriovenous malformation, MMD : moyamoya disease, EDAS : encephalo-duro-arterio-synangiosis, ECA : external carotid artery.
The incidental AVM was managed expectantly as obliteration of the main feeders was anticipated with the future progression of MMD. Follow-up angiography, performed 4.5 years after EDAS, showed progressive stenosis of the right ICA with decreased flow to the AVM (Fig. 1B). The AVM appeared to regress on MRI taken 6.5 years after EDAS but subsequent MRI taken 3 years later showed the increased AVM. Subsequent angiography, performed 9.5 years after EDAS, demonstrated newly-developed AVM feeders from the EDAS site causing no significant change in the size of the AVM (Fig. 1C). Gamma knife radiosurgery (GKS) was performed with a marginal dose of 28 Gy (50% of the maximal dose) considering an increasing steal effect by the persistent AVM. The patient is currently being closely followed with no significant neurological change, and obliteration of the AVM was strongly indicated by MRI taken 2 years after GKS (Fig. 1D).
Case 2
A 7-year-old girl visited our outpatient clinic complaining of TIAs in the left arm and leg for 3 years. Brain MRI did not reveal any parenchymal lesion, but severe ICA steno-occlusion was suspected on MRA. A neurological examination on admission revealed no focal neurological deficit. The patient underwent cerebral angiography, which revealed severe steno-occlusion of the right ICA, ACA, and MCA with abnormal vascular networks (Fig. 2A). The left ICA was relatively unaffected but diffuse stenosis of the left MCA and ACA was observed. There was no arteriovenous shunting at that time. Diamox-enhanced SPECT revealed mild hypoperfusion in the right posterior parietal lobe and decreased vascular reserve in the right frontal, temporal, and parietal lobes. EDAS was performed on the right hemisphere, followed by subsequent EDAS on the left hemisphere. Follow-up MRI and MRA, taken 1 year after EDAS, confirmed the absence of abnormal signal void structures and the presence of transdural collateral vessels from the bilateral EDAS sites. The patient reported no TIAs thereafter but has been lost to follow-up.
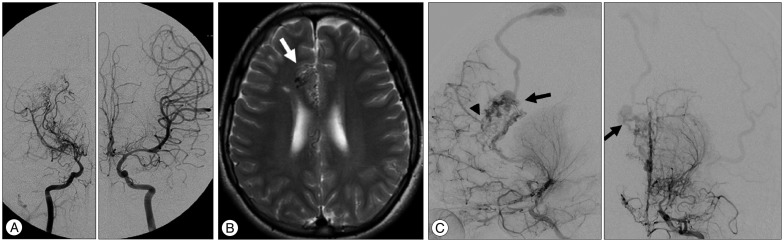
Case 2 images. A : Initial angiography showing steno-occlusive lesions with abnormal vascular networks in the right hemisphere. The left ICA is relatively unaffected. B : Subsequent follow-up MRI taken 8 years after EDAS reveals abnormal signal void structures at the anterior part of the right cingulate gyrus (arrow). C : Follow-up angiography performed 8 years after EDAS, confirming a newly developed AVM with MMD progression. Anomalous arteriovenous shunting (arrow) is more visible on left ICA angiograms. Note the established collateral channels (arrowhead) to the adjacent frontal lobe. ICA : internal carotid artery, EDAS : encephalo-duro-arterio-synangiosis, AVM : arteriovenous malformation, MMD : moyamoya disease.
The patient revisited our outpatient clinic 6 years after the last follow-up for evaluation of incidental lesions on MRI. Although no new infarctions or hemorrhages were identified, abnormal signal void structures were noted at the anterior part of the right cingulate gyrus (Fig. 2B). Follow-up angiography was performed 8 years after EDAS and confirmed a newly developed AVM with MMD progression. The AVM was slowly fed by the reconstituted anterior cerebral artery and drained into the superior sagittal sinus through a single cortical vein (Fig. 2C). The new but asymptomatic AVM is currently being closely followed for more than 2 years without further surgical intervention. Subsequent MRI performed 9.5 years after EDAS did not reveal any significant change.
DISCUSSION
The two cases described herein represent an unusual combination of MMD with AVM. We have reviewed previous case reports of MMD-associated AVMs (Table 1). In most cases, AVM and MMD were diagnosed simultaneously at the time of initial presentation at the hospital1,2,6,7,8,10,11,12,13,15,16), which makes it difficult to evaluate the relationship and their effect on each other. The high-flow stress of an AVM may have resulted in intimal thickening of feeding arteries leading to the moyamoya phenomenon, especially in cases with unilateral (probable) MMD9,12). Some AVMs are supplied by normal cerebral arteries; thus, the coexistence of AVM and MMD was thought to be purely incidental13). These incidental AVMs may be supplied by moyamoya collaterals with MMD progression and by branches of the ECA through naturally developed transdural collaterals at the end-stage of MMD or through surgically established collaterals at the synangiosis or bypass sites as in our first case6,11). Our first case was presumed to be an incidental AVM because such a small size AVM was not expected to elicit a high-flow stress, the AVM was fed by relatively normal arteries, and MMD was less severe in the ipsilateral hemisphere at initial diagnosis.
Rarely, MMD may precede the development of a de novo AVM as in our second case. Three de novo AVMs have been reported until recently3,5,14). One developed in the same region of a previous parietal infarct in an 11-year-old boy14). Another case developed in the medial frontal lobe where SPECT demonstrated decreased perfusion in a 9-year-old girl5). This case is very similar to our second case with respect to AVM location and angiographic appearance. The authors assumed that the hyperangiogenic environment of MMD in combination with the local angiogenic stimulation might have contributed to the development of the new AVM5,14). The last case is a de novo occipital AVM that developed after bilateral revascularization surgery in a 14-year-old girl3). The authors assumed that, because they did not perform extended indirect pial synangiosis and/or an additional burr-hole trephination in the posterior circulation, the initiation of AVM development could not have been due to the iatrogenic arteriovenous fistula. However, the authors did not completely rule out the possibility that the patient initially had a micro-AVM or small arteriovenous fistula before surgery.
A de novo AVM would be better explained by anomalous arteriovenous shunting that developed as a consequence of angiogenic failure11). It has been assumed that increased flow from perforating vessels and their end capillaries could link to normal draining veins which, in turn, became distended and take on the appearance of an AVM8). From the viewpoint of basic pathology, moyamoya disease and an AVM are known to have similar biologic backgrounds of the increased expression of angiogenetic factors such as vascular endothelial growth factor, as well as inflammatory molecules, including tumor necrosis factor-α, interleukin-6, and matrix metalloproteinases3). Development of a de novo AVM, although rare, might have been attributable to the expression of these molecules in moyamoya disease.
As the pathogenesis of AVM associated with MMD is still poorly understood, no solid consensus on optimal treatment has been reached. Surgical removal of the AVM is generally indicated if symptoms arise, but surgical resection poses a significant risk of interrupting the delicate collateral channels to adjacent ischemic brain tissue. Thus, less invasive treatment options such as GKS may be an effective alternative for treating AVM associated with MMD15). In the first case, we initially decided to manage the incidental AVM expectantly, as the main feeder stemmed from the ICA, which we believed would be obliterated with the progression of MMD. But unlike our expectation, new collateral vessels from the ECA formed as the ICA feeder became progressively occluded. The AVM persisted, and GKS was performed considering an increasing steal effect by the persistent AVM. In the second case, however, GKS was considered to have a significant risk of damaging the established collateral channels to the adjacent frontal lobe. In addition, we assumed that the risk of rupture of MMD-associated AVMs would be low given the low flow characteristics. In the reviewed literature, five of the 17 patients with a MMD-associated AVM presented with hemorrhage (Table 1). Two developed basal ganglia hemorrhage close to the AVM location8,11), which might be also attributable to the fragile moyamoya vessels11). The other three had remote hemorrhage from the AVM location. Furthermore, none of the four AVMs with expectant management developed later hemorrhage over follow-ups of 1-6 years5,8,11).
CONCLUSION
The pathogenesis, natural history, and optimal treatment of MMD-associated AVMs are still poorly understood. An incidental AVM diagnosed simultaneously with MMD can be effectively managed with GKS even after surgical revascularization. However, a de novo AVM may be managed expectantly considering the possible risks of damaging established collaterals, low flow characteristics, and probably low risks of rupture.