Leptomeningeal Carcinomatosis of Gastric Cancer Misdiagnosed as Vestibular Schwannoma
Article information
Abstract
Gastric cancer is one of the most common causes of cancer-related death in Asian countries, including Korea. We experienced a case of leptomeningeal carcinomatosis (LC) from gastric cancer that was originally misdiagnosed as vestibular schwannoma based on the similar radiological characteristics. To our knowledge, LC from gastric cancer is very rare. In conclusion, our experience with this case suggests that clinicians should consider the possibility of delayed leptomeningeal metastasis when treating patients with gastric cancer.
INTRODUCTION
Leptomeningeal carcinomatosis (LC) is a metastatic malignant condition that involves the pia mater and arachnoid membrane5). In adult malignancies, where LC occurs most commonly, the most common origin of LC is breast cancer, followed by lung cancer and melanoma, whereas among the pediatric malignancies, leukemia shows the highest incidence of LC14). The prevalence of LC in these malignancies is estimated at approximately 5-8%9). In addition, LC is reported to occur most frequently in malignancies such as breast cancer, lung cancer, leukemia, and lymphoma10). In patients with gastric cancer, however, the prevalence is less than 0.16%. This implies that LC is not a rare disease entity7). In Asian countries, particularly Korea and Japan, the prevalence of gastric cancer is relatively high compared to that in western countries, and the prevalence of LC has also been reported to be high compared with other countries6,11). According to Tsujikawa et al.13), the prevalence of LC in cases of gastric cancer ranges from 1.7% to 5.5% in Japan.
In the case described here, the initial presentation or the location and shape of the lesions on magnetic resonance imaging (MRI) scans were suggestive of bilateral vestibular schwannoma. At that time, the patient was therefore suspected of having Type II neurofibromatosis. However, the patient presented with an aggravation of symptoms during follow up to monitor his clinical course, and the patient was ultimately diagnosed with LC on the basis of brain biopsy. To our knowledge, such cases of LC from gastric cancer are very rare. Here, we describe this case of a patient with LC from gastric cancer and review the relevant literature.
CASE REPORT
A 58-year-old man presented at the outpatient clinic of the department of otolaryngology with a chief complaint of a sustained, sudden-onset bilateral hearing difficulty 4 months previously. The patient was hospitalized for further evaluation and treatment of bilateral sudden sensorineural hearing loss. Both tympanic membranes were intact. A Weber's tuning-fork test revealed no lateralization. A Rinne test was not performed because the patient did not provide consent for this procedure. On pure tone audiometry, the patient had an air conduction threshold and bone conduction threshold of 38 dB and 35 dB, respectively, on the left side and 40 dB and 38 dB, respectively, on the right side. The patient was suspected to have bilateral vestibular schwannoma involving both inner ears based on T1-weighted temporal MRI scans (Fig. 1). We assumed that the patient was less likely to present with symptom aggravation in the short term if he had bilateral vestibular schwannoma. Accordingly, the patient was scheduled to undergo outpatient short-term follow-up examinations, including brain MRI, after 3 months and was discharged. Four months later, at a visit for monitoring of the clinical course, the patient reported bilateral loss of hearing. The patient visited the hospital again with chief complaints of bilateral temporal headache, dizziness, vertigo, and vomiting. On physical examination, the patient showed no notable neurological deficits or neck stiffness in addition to hearing loss. An increase in the size of the lesions in both inner ears was detected on contrast-enhanced temporal MRI, accompanied by the presence of newly developed lesions in the cisternal segments, Meckel's caves, and the left cerebellum (Fig. 2). The patient also complained of lower back pain. Thus, evaluation with whole-spine MRI was done which revealed multiple hyperintense lesions at the L1-L4 level of the spinal cord (Fig. 3).
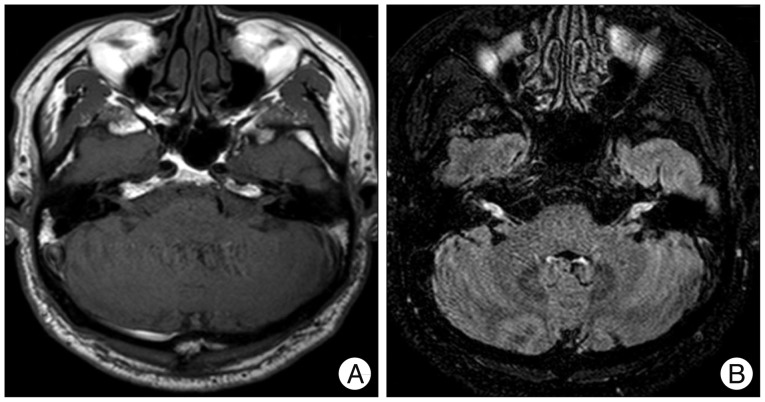
On T1-weighted axial image (A) and a 3-dimensional fluid attenuated inversion recovery VISTA CE one (B), taken at the patient's initial visit due to hearing difficulty, there are multiple enhanced lesions along the cisternal and canalicular segments of the bilateral vestibulocochlear nerves.
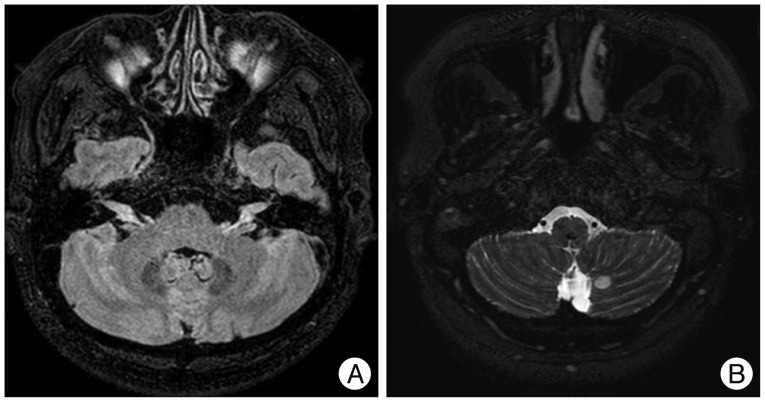
On a 3-dimensional fluid attenuated inversion recovery VISTA CE image (A), there is an interval growth of enhanced areas along the cisternal and canalicular segments of the bilateral eighth nerves and the newly developed intense enhancement of the bilateral trigeminal nerves involving the cisternal segments and Meckel's caves. In addition, on a 3-dimensional DRIVE axial image (B), there is a newly developed, circular enhancing area in the left medial cerebellum.
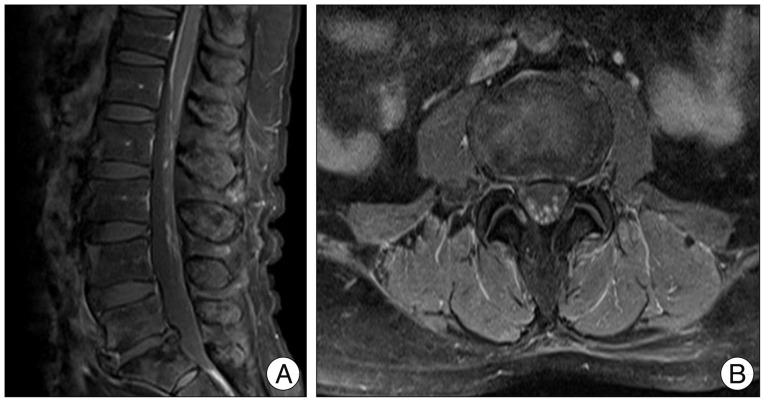
The enhanced magnetic resonance image of the spine. On T1-weighted turbo spin-echo sagittal image (A) and axial image (B), there are multifocal enhanced lesions in the conus medullaris and cauda equine. These findings are suggestive of metastatic tumors.
The patient had been diagnosed with gastric cancer 2 years before the onset of hearing difficulty, for which the he underwent subtotal gastrectomy with gastroduodenostomy and received 6 cycles of anticancer chemotherapy. Thus, it was deemed to be highly probable that the multiple lesions detected on the MRI scans were metastatic lesions. We performed positron emission tomography-computed tomography to investigate the presence of primary lesions at other sites; however, no additional hypermetabolic lesions or metastasis to other organs was detected (Fig. 4). We performed abdomino-pelvic computed tomography to establish recurrent gastric cancer at the primary site as part of the differential diagnosis, but this revealed no notable findings.
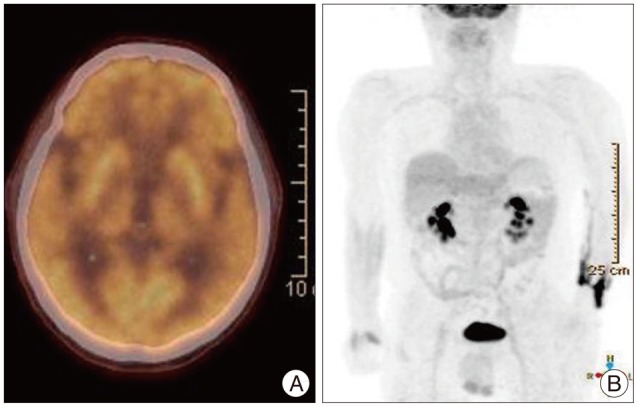
Positron emission tomography-computed tomography. On transverse image (A) of low-dose head CT, there are non-hypermetabolic multiple brain nodules. These findings are suggestive of benign lesion. In addition on torso maximal intensity projection coronal view image (B) of the whole body, there are no metastatic lesions in any other sites.
In order to excise the brain lesions and obtain a histopathologic diagnosis, craniotomy was performed and the tumor was removed via a retrosigmoid approach for the left internal carotid artery (ICA) lesions and those in the cerebellum. We identified a mucoid mass that was approximately 1 cm in diameter (Fig. 5) with intraoperative retraction of the cerebellar hemisphere, and observed that the eighth cranial nerve originated from the brain stem. We performed frozen section biopsy of the mucoid mass, which revealed a diagnosis of metastatic adenocarcinoma. In light of this diagnosis, we discontinued the surgical operation.
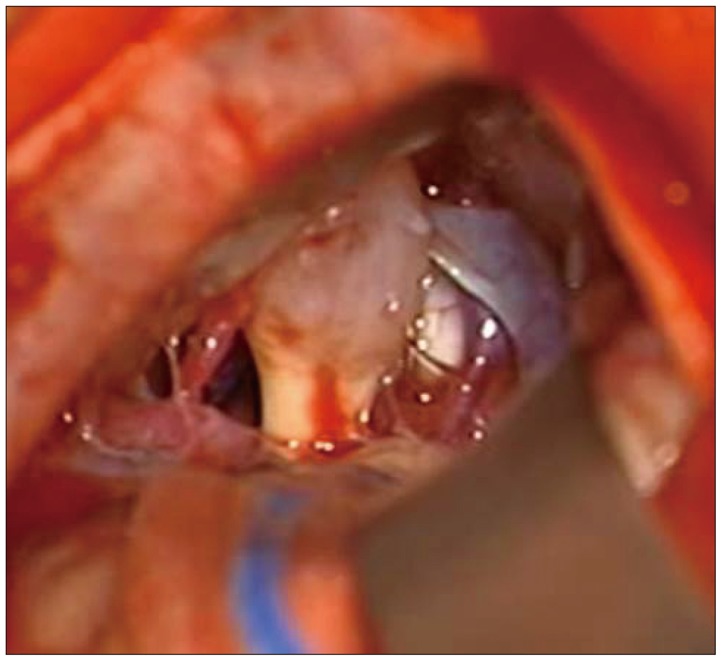
An intraoperative microscopic image shows a whitish, mucoid-like mass of 1 cm in diameter around the vestibulocochlear nerve.
On histopathologic analysis, the cells were found to have peripheral nuclei. Further, there were abundant "signet-ring" cells and discohesive, pleomorphic malignant cells, with the central region of the cells filled with intracytoplasmic mucin vacuoles (Fig. 6). On the basis of these histopathologic findings, the patient was diagnosed with "signet-ring" cell adenocarcinoma, which matched the patient's previous histopathologic diagnosis of gastric cancer prior to surgery 2 years previously.
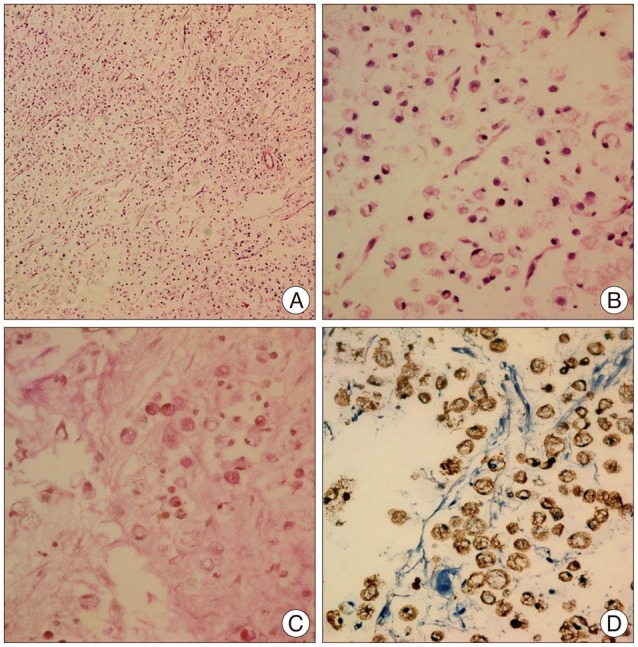
A histopathologic image of the brain tumor shows discohesive, pleomorphic, malignant cells with a signet-ring appearance, with a prominent intracytoplasmic mucin vacuole where the nucleus is peripherally dispersed. (A) Hematoxylin and eosin stain, ×100; (B) hematoxylin and eosin stain, ×200; (C) mucicarmine stain (+), ×200; and (D) CK stain (+), ×200.
The patient's immediate postoperative mental status was defined as drowsy. Additionally, the patient had an overall motor function of Grade IV. He subsequently received whole-brain radiotherapy (RT), with 10 courses at irradiation dose of 3000 cGy in total. Following the completion of RT, his mental status remained stuporous. The patient had a motor function of all extremities Grade II, accompanied by unstable respiratory function. Due to neurological deterioration, the patient was hospitalized in an intensive care unit (ICU), but died from pneumonia 1 week later.
DISCUSSION
Leptomeninges are formed by two membranous structures : the arachnoid membrane and pia mater. Cerebrospinal fluid (CSF) flows between the two membranes and also forms part of the leptomeninges. In some instances, a solid tumor arising in another tissue can directly extend into the CSF, or hematologic cancers, including leukemia, can be seeded into the CSF through hematogenous dissemination. In these cases, the tumor cells disseminate into the nervous system via the CSF. These conditions are collectively termed as LC, which was first reported by Eberth in 1870, although the term LC was later coined in the twentieth century.
On average, LC is diagnosed within 1-year of the diagnosis of the primary cancer. However, since imaging modalities do not aid in diagnosis in many cases, the diagnosis for many patients may be delayed until after the onset of neurological symptoms7). The clinical presentations of LC commonly include headache (85%), nausea/vomiting (58%), and seizure, in decreasing order of frequency7). In the case described in this report, however, the main symptom was hearing difficulty. This symptom has been rarely described in the literature. Additionally, the MRI findings (bilateral ICA mass) and symptoms were suggestive of schwannoma, further delaying the diagnosis.
Currently, there is no established single diagnostic test for LC, although CSF cytology with spinal tapping and MRI are useful in diagnosing LC. Park et al.8) reported that the diagnostic sensitivity of MRI was increased by up to 91% with the additional consideration of positive CSF cytology results, although the sensitivity of CSF cytology alone for LC was at most 54%. CSF aspirated from patients with LC commonly exhibits increased pressure, pleocytosis, elevated levels of protein and lactate dehydrogenase (LDH), and hypoglycemia2).
In the present case, CSF analysis revealed a CSF pressure of 27 mm Hg (normal range : 5-15 mm Hg) and a protein level of 178 mg/dL (normal range : 8.0-43 mg/dL), with both parameters exceeding the normal range. In addition, the glucose level was 71 mg/dL (normal range : 40-70 mg/dL), the LDH level was 18 IU/L (normal range : 18-46 IU/L), and the white blood cell count was 4 mm3 (normal range). Furthermore, no malignant cells were identified on CSF cytology.
Currently, chemotherapy or RT is the standard treatment for patients with LC, although these are performed as a palliative measure rather than as a cure, and the resultant patient outcomes are suboptimal14). Patient prognosis is reported to be poor for LC arising from gastric cancer. Even in patients who underwent chemotherapy and RT following the diagnosis of LC, the mean survival has been reported to be only approximately 4-6 weeks3,4). In 2011, Tomita et al.12) performed a retrospective analysis of 12 patients who were diagnosed with LC from gastric cancer. According to these authors, the mean survival for patients who underwent triple-treatment regimens [chemotherapy, whole brain RT, and ventriculoperitoneal (VP)-shunt operation] was more than 100 days longer that it was for those who received only chemotherapy or chemotherapy combined with whole brain RT, indicating that patients who undergo triple-treatment regimens achieve better outcomes. However, it is important to note that there are not many cases for which triple-treatment regimens have been administered. Therefore, although promising, the VP-shunt operation is not yet an established modality. Moreover, there are currently no standard treatment guidelines for LC1). In the present case, the diagnosis of LC was based on the analysis of the surgical biopsy specimen. Ten days later, the patient underwent whole-brain RT. Two weeks after the initiation of RT, his mental status became stuporous, and the patient died 1 week after admission to the ICU.
CONCLUSION
This case report describes a patient who was diagnosed with LC from gastric cancer. To our knowledge, cases such as this are rare. The current case is notable not only because the patient presented at the outpatient clinic of our medical institution with chief complaints of hearing impairment rather than neurological deficits such as headache, nausea, or seizure, but also because the MRI findings were suggestive of vestibular schwannoma. On the basis of the clinical presentation and imaging findings, the current case seemed to be initially misdiagnosed. RT should have been treated much earlier with proper diagnosis for better prognosis. However, currently, there is no established treatment regimen or highly sensitive test with which a diagnosis of LC from gastric cancer can be made. In conclusion, our case highlights the importance of the development of new diagnostic and treatment regimens for delayed LC which originate from gastric cancer.