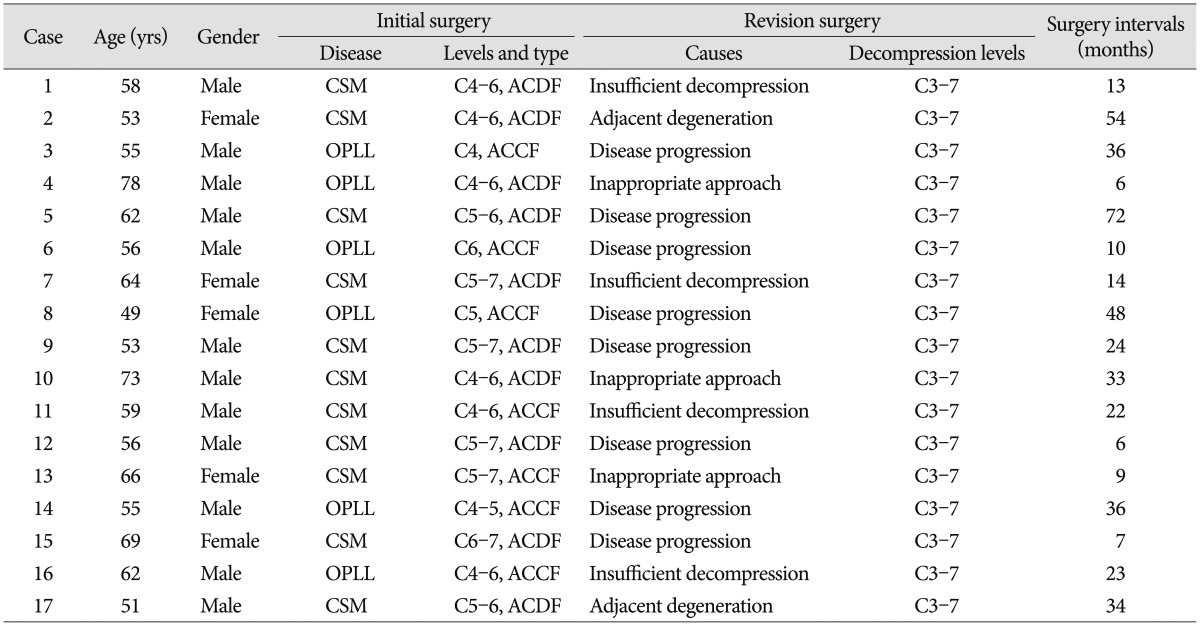
Outcomes of Secondary Laminoplasty for Patients with Unsatisfactory Results after Anterior Multilevel Cervical Surgery
Article information
Abstract
Objective
To investigate the causes for failed anterior cervical surgery and the outcomes of secondary laminoplasty.
Methods
Seventeen patients failed anterior multilevel cervical surgery and the following conservative treatments between Feb 2003 and May 2011 underwent secondary laminoplasty. Outcomes were evaluated by the Japanese Orthopaedic Association (JOA) Scale and visual analogue scale (VAS) before the secondary surgery, at 1 week, 2 months, 6 months, and the final visit. Cervical alignment, causes for revision and complications were also assessed.
Results
With a mean follow-up of 29.7±12.1 months, JOA score, recovery rate and excellent to good rate improved significantly at 2 months (p<0.05) and maintained thereafter (p>0.05). Mean VAS score decreased postoperatively (p<0.05). Lordotic angle maintained during the entire follow up (p>0.05). The causes for secondary surgery were inappropriate approach in 3 patients, insufficient decompression in 4 patients, adjacent degeneration in 2 patients, and disease progression in 8 patients. Complications included one case of C5 palsy, axial pain and cerebrospinal fluid leakage, respectively.
Conclusion
Laminoplasty has satisfactory results in failed multilevel anterior surgery, with a low incidence of complications.
INTRODUCTION
How to choose surgical approach in severe multilevel cervical cord compression case is a hot debate on spine surgery. Some surgeons think anterior surgery should be of the primary choice, because of direct decompression, restoring intervertebral height and reconstruction of stability7,12). Some surgeons prefer posterior approach, owing to easy decompression, low surgical risks and complications14). Whereas other surgeons suggest that combined anterior and posterior approach at one stage achieve more sufficient decompression though with increasing of complications and costs10).
Multiple factors should be considered when a surgery is to be performed, especially on patients with multilevel cord compression from both ventral and dorsal parts. Which approach is better to decompress the cord sufficiently and maintain the spinal column simultaneously, anterior or posterior? However, in clinical practice, surgeon's experience and surgical skills are usually of the primary consideration. Although anterior discectomy and fusion (ACDF), or corpectomy and fusion (ACCF) has satisfactory results in treatment of multilevel lesions of cervical spondylosis and ossification of the posterior longitudinal ligament (OPLL), some patients remain complain of persistence or recurrence of new symptoms. Reports elucidated the causes of unsatisfactory results and the revision techniques have so far been limited. Thus, the purpose of this study was to investigate the causes for failed anterior multilevel ACDF or ACCF, and the outcomes of secondary laminoplasty.
MATERIALS AND METHODS
Study population
Between Feb 2003 and May 2011, 23 patients who complained of persistence of primary symptoms or occurrence of new symptoms for more than 3 months after multilevel anterior cervical surgery (ACDF or ACCF) in our spine surgery center were reviewed in the study. Patients demonstrated as neck pain, cervical movement restriction, spasticity, sensory loss, weakness, or sphincter dysfunction to different extent. Therapeutic treatments included traction, collar support, physical therapy, functional exercise and analgesics. Of 23 patients, 17 without significant neurological improvement underwent secondary laminoplasty (Table 1). The study population consisted of 12 men and 5 women, with a mean age of 59.9±5.6 (range, 49-78) years old. There were 11 cases of spondylotic myelopathy and 6 cases of OPLL. Main indications for the second surgery were when patients displayed signs of cord compression at least at 2 levels associated with/without canal stenosis corresponding to signs of myelopathy. Major exclusion criteria were symptomatic treatment less than 3 months, axial neck pain as the solitary symptom, cervical instability, active systemic infection, and other contraindications in routine surgery. Instability documented as angle difference (Cobb angle) more than 15 degrees and/or listhesis more than 3 mm on extension and flexion lateral views.
Surgical procedures
The patient underwent a general anesthesia and is positioned prone with the head and neck held in slight flexion on the operating table. A cervical midline incision is performed down to the cervical fascia, and the cervical musculature is reflected off of the involved cervical lamina. The decompression levels extended to one level cranial or/and caudal to compressive lesions are included. A 4-mm laminotomy is created just medial to the level of the pedicles on the clinically more symptomatic side, or on the most narrowed side on images, if there is no dominant symptom side. Then the involved ligaments and flavum is removed until the pulse of dura sac is seen. The external cortex is thin out to a 3- to 4-mm trough on the contralateral lamina, again just medial to the level of the pedicles, and inner cortex is preserved. The laminae are lifted carefully to create a greenstick fracture on the hinge side, expanding the spinal canal diameter. Then cervical titanium mini-plate (Medtronic Sofamer Danek, Memphis, USA or Depuy Synthes, Bettlach, Switzerland) is placed at each level secured by two 4-mm screws onto the lamina and two at the level of the lateral masses. At last, the surgical wound is closed in layers. The patient is placed in a hard cervical collar for the first 6 weeks after surgery.
Outcome measures
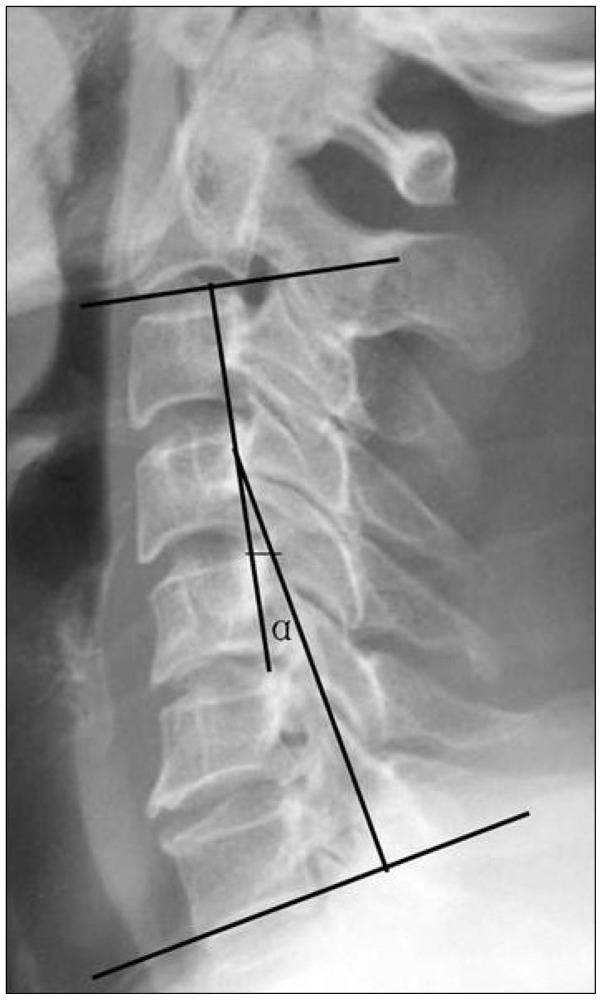
Patients' ages, gender, disease, the first surgical technique and levels, intervals between the first surgery and the onset of new symptoms or the persistence of primary symptoms, re-surgical levels, and causes for revision were examined. The outcomes were evaluated preoperatively, at 1 week, 2 months, 6 months, and the final visit. Neurological status was evaluated by using the Japanese Orthopaedic Association (JOA) Scale. Scores are based on the rating of motor function (fingers, 0 to 4 points; shoulder and elbows, -2 to 0 points; and lower extremity, 0 to 4 points), sensory function (upper extremity, 0 to 2 points; lower extremity, 0 to 2 points; and trunk, 0 to 2 points) and urinary bladder function (0 to 3 points). A normal JOA score is 17 points. Recovery rate was calculated as : (scores at follow up-scores preoperatively)/(17-scores preoperatively)×100%. Recovery rate more than 75% was classified as excellent, 50-75% good, 25-50% fair, and less than 25% poor; the excellent to good rate was calculated. Patients were required to rate their neck pain, using the visual analogue scale (VAS; 0 mm=no pain, 100 mm=worst pain) at different intervals, supplemented with at 1 day postoperatively. Cervical alignment was created by a line parallel to the inferior aspect of the C2 body and a line parallel to that of the C7 body, according to Cobb's method on neutral lateral view (Fig. 1).
Statistics
Average values were presented as mean standard±deviation. Statistical analysis was performed using the analysis of variances analysis or the χ2 test. Significant difference was set at p≤0.05. All data were analyzed by SAS 8.0 (Statistical Analysis System, SAS Institute Inc., Cary, NC, USA).
RESULTS
Mean duration between the first surgery and the onset of new symptoms or persistence of symptoms was 14.5±4.2 (range, 1 to 44) months. Ten patients underwent ACDF and 7 patients ACCF for the first time. Mean first surgical levels were 2.6±0.4 and mean secondary decompression levels were 4.0±0.7. Causes for revision surgery were inappropriate approach in 3 patients (17.6%), insufficient decompression in 4 patients (23.5%), adjacent degeneration in 2 patients (11.8%), and disease progression in 8 patients (47.1%). There were 8 patients of disease progression, with 4 cases of OPLL and 4 cases of cervical spondylotic myelopathy (CSM). The progression levels increased from 1.25±0.2 at the first surgery to 2.25±0.4 at the second surgery on OPLL patients, and from 1.5±0.2 to 2.5±0.4 on CSM patients.
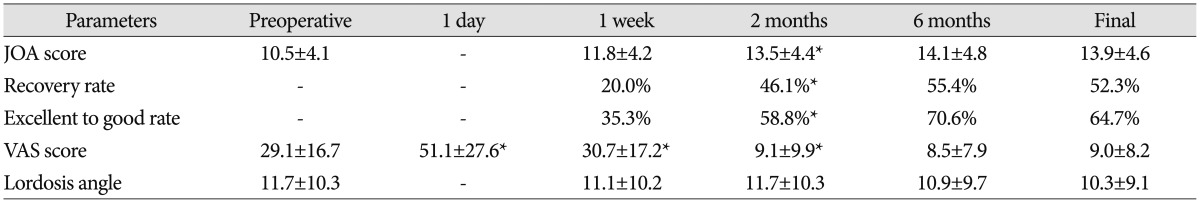
Mean follow-up duration was 29.7±12.1 (range, 6-84) months. Mean JOA score was 10.5±4.1 preoperatively, 11.8±4.2 at 1 week, 13.5±4.4 at 2 months, 14.1±4.8 at 6 months and 13.9±4.6 at the final visit (Table 2); significant improvement achieved at 2 months (p<0.05) and maintained thereafter (p>0.05). Mean recovery rate was 20.0%, 46.1%, 55.4%, and 52.3% after surgery, respectively; significant improvement was detected at 2 months (p<0.05) and maintained with the time (p>0.05). One case had excellent result, 5 good, 7 fair, and 4 poor, with an excellent to good rate of 35.3% at 1 week; two cases had excellent results, 8 good, 5 fair, and 2 poor, with an excellent to good rate of 58.8% at 2 months; three cases had excellent results, 9 good, 4 fair, and 1 poor, with an excellent to good rate of 70.6% at 6 months; three cases had excellent results, 8 good, 4 fair, and 2 poor, with an excellent to good rate of 64.7% at the final visit. The excellent to good rate improved significantly at 2 months (p<0.05). Mean VAS score was 29.1±16.7 preoperatively, 51.1±27.6 at 1 day, 30.7±17.2 at 1 week, 9.1±9.9 at 2 months, 8.5±7.9 at 6 months, and 9.0±8.2 at the final visit. VAS score decreased at 1 week (p<0.05) and at 2 months (p<0.05). Mean lordotic angle was 11.7±10.3° preoperatively, 11.1±10.2° at 1 week, 11.7±10.3° at 2 months, 10.9±9.7° at 6 months and 10.3±9.1° at the final visit; no significance was observed during the follow up (p>0.05) (Fig. 2).
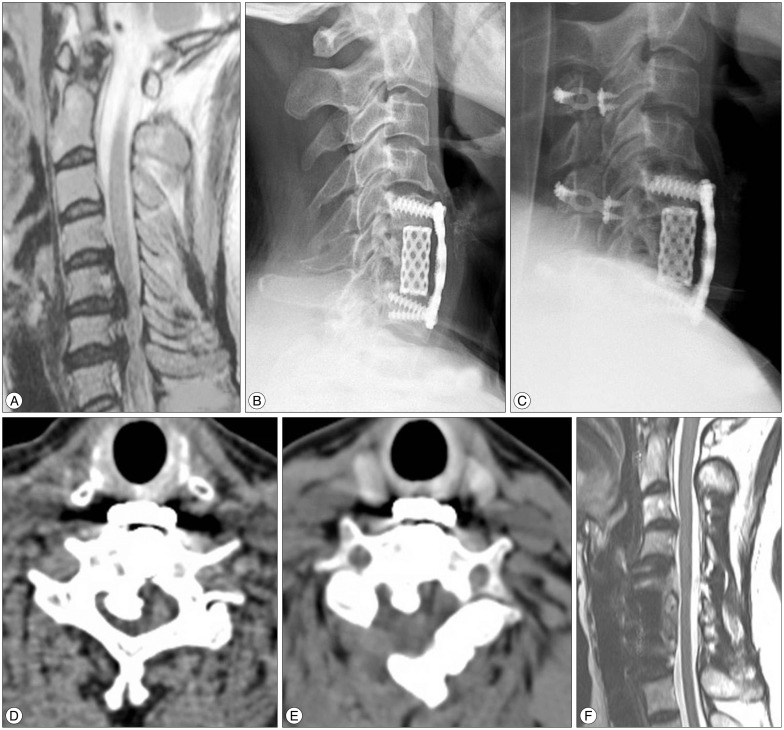
A 51-year-old female with OPLL, whose spinal cord was compressed at levels of C5/6 and C6/7 on T2-weight MRI (A), underwent ACCF (B, lateral view) for the first time. However, the patient complained of persisting numbness and weakness of right limbs. After secondary laminoplasty (C, lateral view), the narrowed canal (D) was enlarged (E) on CT and compression of cord was alleviated (F) on MRI. OPLL : ossification of the posterior longitudinal ligament, ACCF : anterior cervical corpectomy and fusion.
No closure of the 'opening door' was observed. Postoperative complications of the revision surgery emerged in three patients. One case displayed as paralysis of C5 nerve root and alleviated at 6 weeks. One case demonstrated as axial pain and relieved 2 weeks after pain management and physical therapy. One patient complained of cerebrospinal fluid leakage and healed at 1 week.
DISCUSSION
ACDF or ACCF has excellent and good results in treatment of cervical spondylosis and OPLL. The anterior approach removes pathological compression to the cord directly, stabilizes spine column, and maintains cervical alignment. However, if the disease involved multiple levels or posterior compression, the ideal effects of anterior approach are difficult to achieve3,5). It is not easy in performing sufficient decompression and preserving cervical range of motion on ACDF or ACCF. Besides, corpectomy of multilevel vertebrae in series destroys the integrity of spine column and has a risk of nonunion. Especially for patients with congenital or acquired narrowed canal, the compression to cord remains after initial anterior surgery. In the study, 3 patients demonstrated narrowed canal associated with posterior compression and persistent even after aggravation of symptoms after anterior surgery. However, after the secondary laminoplasty, the canal is enlarged significantly and the cord is given more space to breathe1). After a few days, the symptoms all alleviated in these patients. Thus, inappropriate choice of surgical approach is the first cause of failure. If the compression was mainly from the posterior part associated with or without canal stenosis, the posterior approach should be performed.
Second cause was adjacent degeneration. In a study by Matsumoto et al.11) that ACDF patients had significantly higher incidence of progression of disc degeneration than control subjects. Prasarn et al.15) thought biomechanics altered after fusion, with an increased adjacent-segment motion in cervical spine. After fixation and fusion, there is a change in the load of the stiffed fusion which accelerated the degeneration of adjacent disc and endplate, especially on the caudal level. Correspondingly, cord compression by the new protruded materials initiated onset of new symptoms. Faldini et al.6) thought proper lordotic alignment could prevent adjacent degeneration. In our opinion, though factors of degeneration were not systematically elucidated, maintaining lordosis and exercising cervical dorsal musculatures could help a lot.
Third cause was insufficient decompression. It was thought that factors influencing decompression included surgical approach, technique and experience of surgeon, and overall knowledge of pathologic structure. Osteophyte, ossified nucleus pulposus, OPLL and abnormal curvature venous plexus increased complexity of decompression, especially in patients with multilevel lesions or on narrowed canal. Patients with insufficient decompression would have a sooner onset of new symptoms. The last but not the least was disease progression. In the study, half of patients displayed as extension of cord compression though after sufficient initial anterior decompression. The symptoms aggravated gradually until the patients seek for the surgeons. After the revised posterior surgery, the enlarged canal was enough to compensate for the spinal cord in a narrowed space for a long time. Thus, in the study, symptoms of patients with disease progression alleviated immediately after revision. However, there was no exact method to prevent progression. Factors of progression are not clear, which may include metabolism, heredity, endocrine secretion and biomechanics. Choi et al.4) also believed that age, severity of disease, irreversible changes of gray matter in cord, duration of symptoms, and diabetes mellitus took important parts.
Patients' age, general status, extent and range of compression, and safety should be considered, when planning a revision surgery. Pang et al.13) thought although anterior surgery was more effective in relieving cord compromise, inadequate decompression and disease progression might require secondary laminoplasty. Scar tissue around anterior region after anterior surgery increased risks of neurovascular, trachea or esophagus injury. Be-sides, once the cage and vertebrae above and below are fused, it was difficult to remove the protruded or ossified materials along posterior wall of vertebrae, especially in multilevel segments. It was thought that myelopathy could be treated via anterior or posterior approaches, but risks and complications in ACDF increase. Revision surgery via posterior approach could be performed as an excellent alternative17).
Patients after laminoplasty demonstrated significantly neurological improvement. Average JOA score, recovery rate, and excellent to good rate didn't improve until at 2 months, and attained the maximum at 6 months, with a slight deterioration thereafter. Pain relieved at 1 week and didn't aggravate with the time. However, it was reported by Hyun et al.9) that loss of alignment was time-dependent but no further decreased after 18 months. In the study, the lordosis maintained during the entire follow up. In a study by Baba et al.2), excellent to good rate of secondary laminoplasty was 55.6%. They thought that narrowed canal added to the risk of recurrence, and additional laminoplasty could prevent structural compromise occurring adjacent vertebrae. High-signal intensity on cord plays an important role on the prognosis. One case of myelopathy associated with canal stenosis displayed multilevel high-signal intensity. Symptoms and signs had no obvious alleviation, with high-signal intensity remained persistent. Yagi et al.18) also thought that long-term outcome was significantly worse in these patients. The outcome was also worse in patients with expansion of high-signal area, which the causes might be not only from necrosis secondary to ischemia of the anterior spinal artery, but also from the repeated minor traumas inflicted on the cord from an unstable spine. Combining the research and related literatures, we thought that multiple factors affect outcomes of revision surgery, which included age, extent lesion of cord, surgical times, mental disease, and so on. Edwards et al.5) deemed that both corpectomy and laminoplasty arrested progression in multilevel myelopathy, which resulted in significant neurologic recovery and pain reduction. However, the laminoplasty cohort required less pain medication than did the corpectomy cohort. Given the higher prevalence of complications in anterior approach, it was believed that laminoplasty might be the preferred treatment in the absence of preoperative kyphosis. However, although it is a safe and effective technique, complications include paralysis of C5 nerve root, axial pain, and instability of spine, cerebral spinal fluid leakage and close of "opening door"16). However, complication rate is low and will disappear in a short term. Laminoplasty preserving the C7 muscle attachment is very important. In the study, except for the decompression extending to T1, all the other cases preserve muscle insertions into the C7 spinous process. It was also reported that this preservation was associated with a significantly decreased frequency of postoperative axial neck pain8).
Limitations were a retrospective design, small number of patients and short term study. Future studies will seek to research in a prospective, randomized and controlled way with a longer duration. Despite limitations, laminoplasty should be considered as a primary procedure or as a revision procedure versus anterior approach in multilevel lesions because of effective decompression, preservation cervical alignment and minimum morbidity.
CONCLUSION
Laminoplasty has satisfactory results in failed anterior surgery, with a low incidence of complications.
Acknowledgements
The study was financially supported by the National Natural Science Foundation of China (81071450 and 81371930) and Key Talented Man Project of Jiang Su Province (RC2011102).