Radicular Pain due to Subsidence of the Nitinol Shape Memory Loop for Stabilization after Lumbar Decompressive Laminectomy
Article information
Abstract
A number of dynamic stabilization systems have been used to overcome the problems associated with spinal fusion with rigid fixation recently and the demand for an ideal dynamic stabilization system is greater for younger patients with multisegment disc degeneration. Nitinol, a shape memory alloy of nickel and titanium, is flexible at low temperatures and regains its original shape when heated, and the Nitinol shape memory loop (SML) implant has been used as a posterior tension band mostly in decompressive laminectomy cases because the Nitinol implant has various characteristics such as high elasticity and a tensile force, flexibility, and biological compatibility. The reported short-term outcomes of the application of SMLs as posterior column supporters in cervical and lumbar decompressive laminectomies seem to be positive, and complications are minimal except for the rare occurrence of pullout and fracture of the SML. However, there was no report of neurological complications related to neural compression in spite of the use of the loop of SML in the epidural space. The authors report a case of delayed development of radiating pain caused by subsidence of the SML resulting epidural compression.
INTRODUCTION
Spinal fusion with rigid fixation has been the mainstay of surgical treatment for low back pain or instability. However, despite the improvement in radiological fusion rates, the problems related to rigid fixation such as deterioration of the adjacent segment, stiffness of the back, and mechanical failure have been reported7,8,9,10). To overcome these problems, the concept of soft fixation or dynamic stabilization has been advocated7,9,10).
Nitinol is an alloy of nickel and titanium that belongs to a class of materials called shape memory alloys. The Nitinol implant has high elasticity and flexibility (below 10℃) or rigidity (above 30℃) according to the temperature2,3,5). Several spinal surgeons have tried to apply this mechanical property of Nitonol as a posterior column supporter or posterior tension bands in the correction of scoliosis, atlantoaxial instability, traumatic cervical instability, and in degenerative lumbar spinal surgery1,4,6,9,12). The reported complications or problems related to shape memory loops as a posterior column supporter are few in numbers, as the reports dealing with shape memory loops are limited in number. Kim et al.6) reported two cases of memory loop fracture and two cases of pullout in their 1-year follow-up study of 194 patients implanted with loops for the treatment of lumbar disc disorders. However, there has been no report on the risk of neurologic deterioration or pain after to implantation of shape memory loops (SMLs)4,5,6,12). We describe a case of severe radicular pain caused by dural compression following the implantation of loops for posterior column support after L4/L5 decompressive laminectomy.
CASE REPORT
A 51-year-old female patient presented with severe radiating pain bilaterally in the posterior aspect if the thighs and legs of 3 months' duration. Six months prior to admission, she underwent an L4/L5 decompressive laminectomy with SML augumentation from L4 to L5 for chronic right leg radiating pain (L5 distribution) of 6 months' duration. The postoperative course was uneventful and the radiating pain in her right leg was relieved.
However, left buttock pain radiating to the left thigh and leg developed gradually 3 months postoperatively and pain in the right thigh and leg subsequently reccurred. Her bilateral thigh and leg pain did not respond to restriction of activity and maximal medications including ultracet, tramadol, gabapentin (up to 1800 mg a day), and oxycodone (20-40 mg a day). Application of transdermal fentanyl caused dizziness and abdominal discomfort. Repeated root blocks (S1) and epidural blocks were effective only for several hours. A postoperative magnetic resonance imaging (MRI) taken at the time of pain recurrence showed a signal artifact along the SML and no evidence of recurrent disc herniation or lateral stenosis (Fig. 1). She was referred to the authors for further evaluation and management.
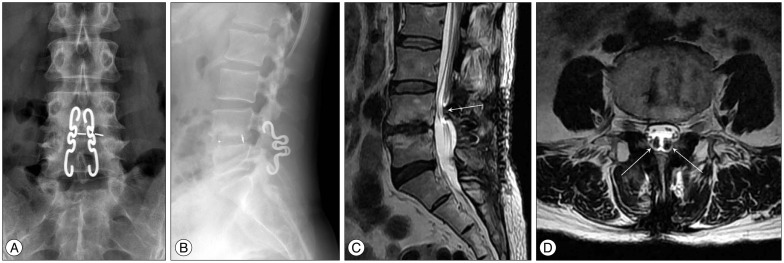
Radiologic findings of the patient. A : An anterior-posterior X-ray of the lumbar spine showing the placement of the shape memory loop and the posterior lumbar interbody fusion at the level of L4/5. B : A lateral X-ray of the lumbar spine. C : A sagittal T2-weighted image showing the encroachment of the shape memory loop (arrow) on the dural sac in the upper lamina of L4 and the lower lamina of L5. D : An axial T2-weighted image at the upper lamina level of L4.
On examination, no motor weakness or sensory disturbance was detected. The deep tendon reflexes were intact and there was no urinary incontinence. Mild limitation in straight leg raising (60/60) was noted. There was no low back pain. Her pain, much more severe in the left side, occurred bilaterally in the lower buttock and radiated to the posterior thigh and legs along the S1 dermatomes. The radiating pain was aggravated with standing, walking and straining. Careful reading of the MRI taken at the time of recurrence suggested a possible dural compression from the SML in the opinion of the authors, and a computed tomography (CT) myelogram was performed. On CT myelography, the upper and lower distal ends of the SML bilaterally were found to encroaching on the posterior dural sac (Fig. 2), and removal of the SMLs was planned to relieve the bilateral leg pain after receiving informed consent.
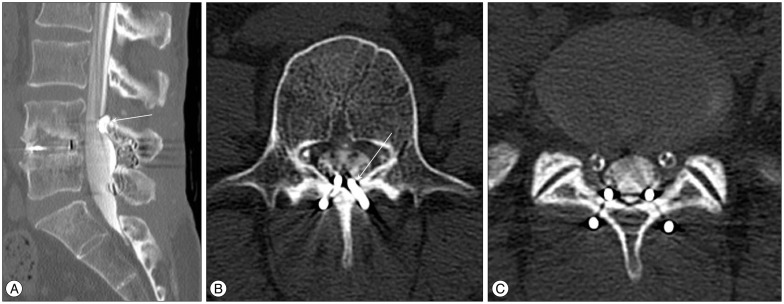
A : A sagittal reconstruction view of the CT-myelogram showing subsidence of the shape memory loop (arrow). An axial view of the CT-myelogram at the level of L4 (B) and L5 (C).
After re-opening of the previous operation site, the paraspinal muscles and fascia were dissected and retracted. The adhesions along the SML were carefully divided and the SMLs were exposed (Fig. 3A, B). It seemed that the SMLs were firmly attached to the spinous processes and laminae, and no jolting movement of the SML was elicited by shaking with Kelly clamps. The SML could not be rotated or bent with several kinds of clamps, so the arms of the SML were drilled down with a diamond burr under microscopic vision (Fig. 3C). The lower ends of the bilateral limbs of the SML could be pushed out after the drilling.

A : An intraoperative photograph showing the placement of the shape memory loop. B : An intraoperative photograph showing the status of the upper limb of the shape memory loop at the level of L4 (right side). C : Drilling of the shape memory loop with a diamond burr. D : A photograph showing the presence of an epidural hole (arrow) made from the previous placement of the limb of the shape memory loop, causing dural compression.
However, the upper ends that were hooked in the upper L4 laminae could not be extracted manually and additional drilling of the upper medial part of the laminae was needed to create rooms for the movement of the hooked SML ends (Fig. 3D). Upon removal of the ends of the SML, a small hole for the SML was noted (Fig. 3D). We noticed that the ligamentum flavum which should be removed to prevent dural compression during application of the SML had been left intact. After securing hemostasis, the overlying wounds were closed in layers.
The postoperative course was uneventful. Her disabling, bilateral radiating pain was relieved 2 days after the operation and the medications could be withdrawn two weeks postoperatively. No symptoms related to possible instability of the laminectomized segment resulting from the removal of the SMLs were noticed until 6 months after the removal of the SMLs.
DISCUSSION
Nitinol shape memory loops as a posterior column supporter
Nitinol, a shape memory alloy of nickel and titanium, is flexible at low temperatures and regains its original shape when heated11). Some spinal surgeons have focused on this characteristic and have tried to apply it to the correction of scoliosis7,9,11). The shape memory loop implant (KIMPF-DI fixing system; CJSC KIMPF Co., Seoul, Korea) is made from Nitinol5,6). The use of the SML implant in the degenerative lumbar spine stems from the pioneering work of Kim et al.6) who advocated its use as a posterior tension band or posterior column supporter, mostly in decompressive laminectomy cases.
Because SMLs have the property of easy application on account of its heat-dependent plasticity and its recovery of the original shape, they seem to have some advantages, including a short operating time, less blood loss, and elimination of the need for wide muscle dissection for pedicle screw fixation6).
Subsidence of shape memory loops
Subsidence of the bilateral limbs of the SML was thought to be the cause of the radiating pain in this case. The reason why we thought the subsidence of the SML limbs generated the pain was the later occurrence of the radiating pain. If the SML itself had caused dural compression after application, the radiating pain should have occurred soon after the initial operation. Indeed, the radiating pain in her right leg was relieved with the first operation (laminectomy with SML application) and she was pain-free for 3 months after the initial operation. The late occurrence of the bilateral leg pain suggested to us that there was bilateral subsidence of the SML because SMLs have been reported to have the characteristics of high elasticity and tensile force3). Furthermore, the new radiating pain did not show the characteristics of neuropathic pain which would have occurred of an injury to the nerve root during operation.
Indeed, it seems difficult for us to assess the exact position of the limbs of the SML with simple X-ray films unless a pullout of the SML occurs. Even, the MRI taken to seek the cause of the pain was not read accurately by the operating surgeon and the referring doctors because the signals around the SML that was encroaching on the posterior dural sac were mis-interpretd as artifacts of the metals which are commonly seen in the postoperative placement of pedicle screws (Fig. 1C). Until now, there has been no systematic, radiological description of the MRI findings of the artifacts of SMLs. A CT-myelogram seems to be very helpful in the evaluation of possible late dural compression by the subsidence of SMLs.
Complications of shape memory loops
The reported complication associated with the application of SMLs is rare. To our knowledge, there were only two cases of pullout and two cases of SML fractures in a study of the 1-year follow-up of 104 patients in lumbar disc disorders6). There was no report describing the pain and neurologic deficits related to dural compression after SML application. The scarcity of the reports of the complications related to SMLs may stem from the rarity of the long-term results of SMLs in degenerative lumbar spine disorders.
Indeed, in the report by Kim et al.6), most of the SMLs (184 of 194 patients) were used in posterior lumbar interbody fusion (PLIF) using a BioFlex System (Biospine, Inc., Seoul, Korea) consisting of titanium pedicle screws and a Nitinol rod. Only 18 patients underwent a SML-only application, and 5 out of 18 patients had SML-only application after decompressive laminectomy. Because the number of the cases (loop only) is too small, it is difficult to draw any conclusions about the relationships between subsidence and SMLs, and it is also uncertain that subsidence and dural compression might be prevented with a combined application of both PLIF and SML.
According to Kim et al.6), the SML was designed for the surgical treatment of L4/5 stenosis, and they stressed the importance of complete removal of the ligamentum flavum to prevent a possible mass effect of the remained ligamentum flavum during the shortening phase of the interspinous distance that occurs with SML application. They also stressed a very minimal skeletonization of the lamina or spinous process of L3 at its upper end and L5 at its lower end and careful determination of where the fixating parts were to be applied. It is uncertain that more medial placement of the limbs of the SMLs would prevent the subsidence of the SMLs. In our opinion, too excessive shortening of the SML might increase the risk of subsidence.
CONCLUSION
Nitinol shape memory loops have been advocated as posterior dynamic stabilizers in degenerative lumbar diseases. It seemed that the short-term results of the SMLs were promising and neurologic complications with SML application have not been reported until now. However, we describe a delayed occurrence of disabling pain resulting from subsidence of the SML limbs causing dural compression in a patient with degenerative lumbar disorder, which necessitated the removal of the SMLs.