Association of Carotid Intraplaque Hemorrhage and Territorial Acute Infarction in Patients with Acute Neurological Symptoms Using Carotid Magnetization-Prepared Rapid Acquisition with Gradient-Echo
Article information
Abstract
Objective
The purpose of our study was to assess prevalence of carotid intraplaque hemorrhage (IPH) and associations between territorial acute infarction and IPH on magnetization-prepared rapid acquisition with gradient-echo (MPRAGE) in patients with acute neurologic symptoms.
Methods
83 patients with suspected acute neurologic symptoms were evaluated with both brain diffusion weighted imaging (DWI) and carotid MPRAGE sequences. Carotid plaque with high signal intensity on MPRAGE of >200% that of adjacent muscle was categorized as IPH. We analyzed the prevalence of IPH and its correlation with territorial acute infarction.
Results
Of 166 arteries, 39 had a carotid artery plaque. Of these arteries, 26 had carotid artery stenosis less than 50%. In all carotid arteries, MR-depicted IPH was found in 7.2% (12/166). High-signal intensity on DWI was found in 17.5% (29/166). Combined lesion with ipsilateral high-signal intensity on DWI and IPH on carotid MPRAGE sequence was found in 6 lesions (6/166, 3.6%). Of patients with carotid artery plaque, MR-predicted IPH was found in 30.8% (12/39) and match lesions with high-signal intensity on DWI and MPRAGE was found in 15.4% (6/39). MR-predicted IPH was significantly higher prevalence in high-grade stenosis group (p=0.010). Relative risk between carotid MPRAGE-positive signal and ipsilateral high-signal intensity on DWI in arteries with carotid artery plaques was 6.8 (p=0.010).
Conclusion
Carotid MPRAGE-positive signal in patients was associated with an increased risk of territorial acute infarction as detected objectively by brain DWI. The relative risk of stroke was increased in high-grade stenosis categories.
INTRODUCTION
Carotid atherosclerotic disease is a major risk factor for stroke and a major of systemic plaque burden2,6,8,13). The progression of carotid atherosclerosis is a complex and often longstanding phenomenon. Also, the progression of carotid atherosclerosis can be contributed to variable factors as followed; systemic factors such as age, sex, and statin therapy and local factors such as intraplaque hemorrhage (IPH), ulcer, and thin fibrous cap6,17,19,20,21). In multiple trials using 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors or statins, reduction in plaque burden as measured by intima-media thickness is modest and slow as compared to improvement in clinical outcome2,6,8,11,12,17). Complex carotid plaques included IPH, ulcer, or thin-fibrous cap were associated with the occurrence of subsequent cerebrovascular events10,22,23). Especially, IPH into the carotid atherosclerotic plaque is significantly associated with more rapid progression in wall and lipid-rich necrotic core (LRNC) size, as well as more rapid progression in luminal stenosis20).
High-resolution MRI, as a noninvasive imaging tool, has proved to be a modality with excellent capability for discriminating tissues of the carotid plaque, including the status of the fibrous cap, LRNC, calcium, and hemorrhage10,19,20,21,22,23,27). T1-weighted MR sequences and three-dimensional (3D) time-of-flight (TOF) angiography are commonly used to detect IPH24,25,26). Far more accurate detection of IPH is now accomplished using advanced, heavily T1-weighted techniques including the magnetization-prepared rapid acquisition with gradient-echo (MPRAGE) sequence. The MPRAGE sequences for detection of IPH using histological confirmation has demonstrated significantly higher sensitivity and specificity compared with the conventional T1 or TOF sequences9). McNally et al.7) reported that in the workup of acute stroke, carotid MPRAGE-positive signal was associated with an increased risk of territorial cerebral ischemic events as detected objectively by brain diffusion tensor imaging. The relative risk of stroke was increased in all carotid stenosis categories but was most elevated in the mild stenosis category. However, this study has only documented the relationship between MPRAGE-positive signals and cerebral ischemic events in Western populations.
The purpose of our study was to assess prevalence of IPH and associations between territorial acute infarction and IPH on MPRAGE in patients with acute neurologic symptoms.
MATERIALS AND METHODS
Patients
This study was conducted with Institutional Review Board approval. Informed consent was obtained from all patients. From April 2012 to January 2013, 136 patients who underwent stroke MR protocol because of acute neurologic symptoms were imaged with the additional carotid MPRAGE sequences. Of these patients, 22 were excluded due to poor image quality and 31 were excluded from the analysis : 1) co-existent >50% ipsilateral intracranial artery stenosis or luminal irregularity (n=13); 2) non-atherosclerotic vasculopathy, such as dissection or moyamoya disease (n=3); 3) evidence of cardioembolism such as atrial fibrillation or previous stroke history of cardiac origin (n=15). 166 arteries of 83 patients (61 males, 22 females; age range 59-89, median age 69 years-old) are eligible for data analysis. Main neurological symptoms of these patients were as fallows; 21 dizziness, 14 dysarthria, 37 unilateral weakness, 9 mental change, and 2 hyperesthesia.
Stroke MR and MPRAGE sequence
All patients with acute neurological symptoms underwent the baseline brain CT scans for evaluating intracranial or subarachnoid hemorrhage in the emergency department. MR examination was obtained with an Achieva 3.0-T Scanner (Philips Medical Systems, Best, the Netherlands) with a 16-channel head coil. Stroke MR imaging was performed immediately following CT scanning with the following techniques : 1) diffusion-weighted imaging (DWI); 2) 3D TOF MR angiography (MRA) of the intracranial arteries; 3) susceptibility-weighted imaging (SWI); 4) perfusion-weighted imaging (PWI); and contrast-enhanced MRA for evaluation of carotid artery. We also performed the MPRAGE sequence for this study. Total scan time was approximately 20-30 minutes.
DWI was conducted with a spin-echo-type echo-planar imaging sequence, with 3 b-values of 0, 500, and 1000 sec/mm2 along all 3 orthogonal axes : repetition time (TR)/echo time (TE)=3000/80 ms; flap angle (FA)=90°; sensitivity encoding=3; field of view (FOV)=220×220 mm; matrix=128×128; section thickness/gap= 5 mm/30%; scanning time=35-38 seconds. Axial dynamic gradient-echo echo-planar PWI was performed after tracking a bolus of 0.03 mmol/kg of gadofosvesettrisodium (Vasovist, Schering, Berlin, Germany). Acquisition parameters were as follows : TR/TE=1850/35 ms; FA=40°; FOV=230×230 mm; matrix= 132×132; and section thickness/gap=5 mm/30%. After image reconstruction and processing, PWI data were transferred to a workstation (ADW 4.2; GE Healthcare, Milwaukee, WI, USA). For MRA, 3D multislab TOF-MRA from the petrous portion of the internal carotid artery (ICA) was generated with the following parameters : TR/TE=23/3.45 ms; FA=20°; FOV=200×200 mm; matrix=488×249; sensitivity encoding factor=2.5; slice thickness=1.2 mm; echo train length (ETL)=1; and number of average=1. We also performed contrast-enhanced carotid MRA from the aortic arch to whole brain by using coronal planes with a 3D spoiled gradient echo sequence optimized for high spatial resolution and an intravenous bolus injection of 0.03 mmol/kg of gadofosvesettrisodium (Vasovist, Schering, Berlin, Germany), which was administered with an automatic injector at a rate of 1 mL/s through an 18-gauge cannula placed in the antecubital vein of the right arm, followed by 15 mL of saline solution, and the following imaging parameters; TR/TE=4.9/1.7 ms, flip angle=27°, slice thickness=1.0 mm, matrix size=384×384, voxel size=1×1×0.7 mm.
For 3D MRPRAGE sequence, segmental acquisition was performed with the sequence TR, the inversion preparation time (TI), and the phase encoding order of the MPRAGE sequences adjusted to optimally identify IPH as hyperintense. Image parameters were as follows : TR/TE/TI=8.7/5.3/304 ms, FA=15°, ETL=32, FOV=140×140 mm, matrix=216×192. Images were obtained 20 mm below the carotid bifurcation to 20 mm above the carotid bifurcation at 1.0-mm slice thickness. The TI was chosen relative to the phase encoding acquisition to maximize contrast between hemorrhage and inflowing blood. Chemical fat saturation was used.
Image analysis
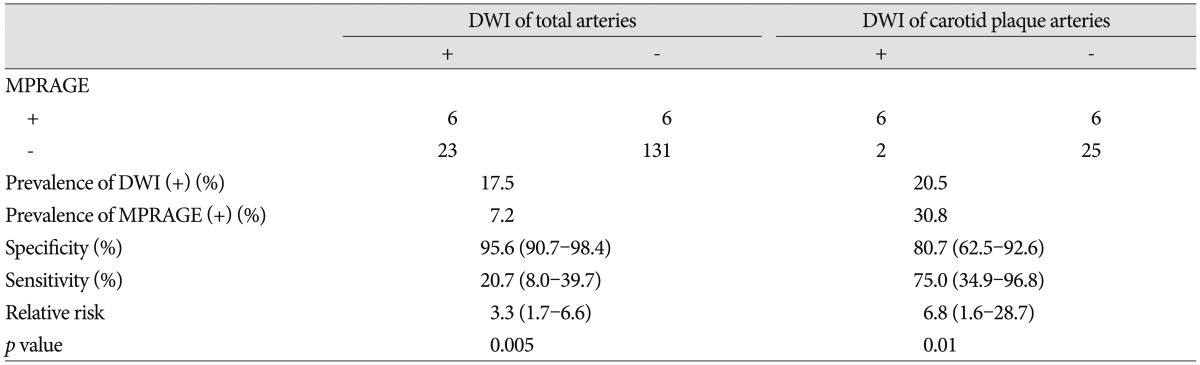
A positive signal on MPRAGE sequence was defined by the location in the plaque and at least 2 image slices with at least 2-fold higher signal intensity compared to adjacent muscle (Fig. 1). Two experienced neuroradiologists determined MPRAGE status in this objective manner independent of brain MR findings. Consensus interpretation was used for the final analysis when interpretation differed. Also, the presence of carotid plaque and stenosis was calculated by one neuroradiologist using the North American Symptomatic Carotid Endarterectomy Trial criteria. Carotid plaque was categorized into 1-49% and ≥50% stenosis.

A 65-year-old woman with an acute infarction of the right basal ganglia. A : A positive signal on magnetization-prepared rapid acquisition with gradient-echo image is defined by the location in the plaque and at least 2 image slices with at least 2-fold higher signal intensity compared to adjacent muscle (arrow). B : Diffusion-weighted imaging (DWI) positive is defined as hyperintense signal on DWI trace with associated decreased signal on the apparent diffusion coefficient map, corresponding to an acute ischemic event at the time of the scan (arrow).
DWI positive was defined as hyperintense signal on DWI trace with associated decreased signal on the apparent diffusion coefficient map, corresponding to an acute ischemic event at the time of the scan (Fig. 1). Acute territorial ischemic events were first classification based on distribution (ipsilateral ICA territory, ipsilateral basal ganglia, and posterior circulation). Only DWI positive events in the ipsilateral ICA territory were placed in the DWI-positive category. DWI images were interpreted by an experienced neuroradiologist blinded to the carotid MPRAGE results.
Statistical analysis
Statistical analysis of carotid MPRAGE signal and brain DWI signal was performed using Fisher exact tests. Likelihood ratios from the 2×2 table were used to determine relative risk of a cerebrovascular ischemic event in MPRAGE-positive lesion with a 95% confidence interval.
RESULTS
Of 166 arteries, 39 (23.5%) on contrast-enhanced MRA and MPRAGE sequence had a carotid artery plaque. Mean percentage of carotid artery stenosis is 30% (range, 5-99%). Of 39 arteries with carotid atherosclerotic plaque, 26 (66.7%) had carotid artery stenosis less than 50% and 13 (33.3%) had stenosis above 50%. In all carotid arteries, MPRAGE-positive IPH was found in 7.2% (12/166). High-signal intensity on DWI was found in 17.5% (29/166). Combined lesion with ipsilateral high-signal intensity on DWI and MPRAGE-positive IPH was found in 6 lesions (3.6%).
Data of the MPRAGE-positive IPH and positive DWI in patients with carotid atherosclerotic plaque show in Table 1. Of 39 arteries with carotid artery plaque, MPRAGE-positive IPH was found in 30.8% (12/39) and match lesions with high-signal intensity on DWI and MPRAGE-positive IPH was found in 15.4% (6/39). MPRAGE-positive IPH was significantly higher prevalence in high-grade stenosis group (p=0.010). Also, matched lesions between ipsilateral positive DWI and MPRAGE-positive IPH was significantly higher prevalence in high-grade stenosis (p=0.018).
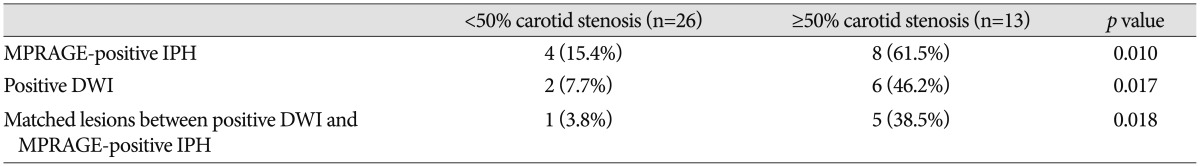
Data of the MPRAGE-positive carotid IPH and positive DWI in patients with carotid atherosclerotic plaque
Data of the relationship of MPRAGE-positive IPH with territorial DWI-positive acute stroke events shows in Table 2. MPRAGE-positive carotids were associated with increased risk of a DWI-positive acute territorial stroke event (Fig. 2). The relative risk of an acute territorial stroke event with an MPRAGE-positive carotid compared with an MPRAGE-negative carotid was 3.3 (p=0.005). The relative risk of an acute territorial stroke event with an MPRAGE-positive carotid in patients with carotid atherosclerotic plaque compared with an MPRAGE-negative carotid was 6.8 (p=0.01).
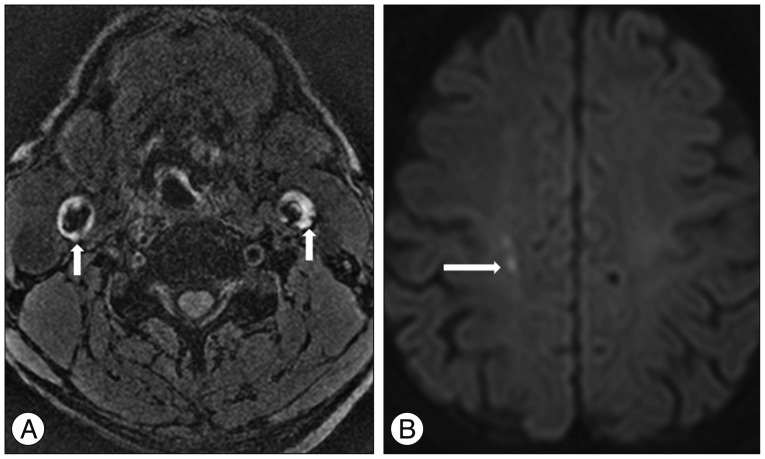
A 73-year-old man with an acute infarction of the right periventricular white matter. A : Magnetization-prepared rapid acquisition with gradient-echo (MPRAGE) image shows the bilateral high signal intensity in the carotid atherosclerotic plaque (arrows). B : Diffusion-weighted image (DWI) shows the diffusion restriction in right periventricular white matter (arrow). MPRAGE-positive carotid artery coupled with territorial stroke events on DWI.
DISCUSSION
This study used MRI at 3-T to immediately undertake combined carotid MPRAGE sequence and stroke MR protocol after admission at emergency room in patients with acute neurological symptoms. In this study, matched lesions between ipsilateral positive DWI and MPRAGE-positive IPH were significantly higher prevalence in high-grade stenosis. The relative risk of an acute territorial stroke event with an MPRAGE-positive carotid compared with an MPRAGE-negative carotid was 3.3-fold. The relative risk of an acute territorial stroke event with an MPRAGE-positive carotid in patients with carotid atherosclerotic plaque compared with an MPRAGE-negative carotid was 6.8-fold.
Early determination of the etiologic factors of acute ischemic stroke is essential for secondary prevention because the risk of recurrence is highly dependent on the underlying cause6). Major identified causes of acute ischemic stroke are extra- or intracranial atheroma, cardioembolic sources, microvascular disease, aortic arch atheroma, and cryptogenic factors. Most hospitals about patients with acute stroke performed stroke MRI after initial brain CT at admission of emergency room. Stroke MRI had been demonstrated to be more sensitive for the detection of acute ischemia and more specific for delineation of infarction core volume when compared to CT3). Recently, some studies reported brain MR examination combined sequences of carotid plaque characterization such as MPRAGE, or conventional plaque MR and the relationship between vulnerable carotid plaque and territorial stroke event5,7). This study was performed the relationship between territorial acute ischemic stroke and carotid IPH using acute stroke MR examination with MPRAGE sequence.
Although IPH in the carotid atherosclerotic plaque is significantly associated with more rapid progression in luminal stenosis20), the association of hemorrhage and symptoms remains a controversial subjects4,5,14,16,19). Sitzer et al.16) demonstrated that ulceration and luminal thrombus were the main sources of downstream cerebral microemboli in patients with high-grade internal carotid stenosis. Kolodgie et al.4) found evidence that showed a hemorrhage might represent a potential atherogenic stimulus. Symptomatic groups had higher prevalence of complex (American Heart Association type IV) plaques on carotid plaque MR5,14). Especially, fibrous cap rupture was associated with increases in DWI lesion in the brain5). Takaya et al.21) reported that the presence of IPH or fibrous cap rupture was significantly associated with subsequent carotid cerebrovascular event. Some from carotid endarterectomy (CEA) studies have supported the relationship of carotid IPH with acute stroke. Spagnoli et al.18) found that IPH in CEA specimens corresponded with a recent clinical stroke or transient ischemic attack based on the presence of neurological symptoms.
Early identification of patients with IPH is very important for decrease of future sequela and optimizing management. The development of IPH posed an immediate and long-term promoting effect on plaque progression. IPH seems to alter the biology and natural history of carotid atherosclerosis20). High-resolution MR imaging is recognized as a reliable method for the comprehensive characterization of atherosclerotic plaque tissue composition and the identification of IPH in particular10,21,22,23,27). T1-weighted MR sequences are commonly used to detect IPH owing to the degradation of hemorrhage into methemoglobin, which results in T1 shortening and correspondingly causes high signal intensity on T1-weighted MR images. Among the T1-weighted MR sequences, black blood T1-weighted fast spin-echo and 3D TOF angiography are currently used for clinical examination. Our study used MPRAGE because recent research has shown this sequence has higher sensitivity and specificity in detecting hemorrhage compared with black blood T1-weighted fast spin-echo and TOF angiography9). The MPRAGE sequence combined with contrast-enhanced T1-weighted and TOF angiography is more practical for many institutions for evaluation of carotid plaque components included IPH. In the current study, the heavily T1-wighted signal of the MPRAGE sequence was accomplished by a magnetization preparation inversion pulse1,28). From signal simulation that incorporated T1 values of muscle, blood, and hemorrhage, the segment TR and T1 in the MPRAGE sequence were selected to suppress the flowing blood signal and enhance tissues with short T1 relative to muscle.
Current studies reported that carotid plaque MR with multiple sequences has used in the workup of acute stroke in a one week after symptom onset5,10). In situ type VI plaque identified by carotid MR imaging was associated with ipsilateral acute transient ischemic attack or ischemic stroke. The prevalence of IPH at baseline in Western population was 49.0% and the presence of IPH was associated with a 6-fold higher risk for events15). Also, the annualized event rate in subjects with detectable IPH was 17.71% compared with 2.43% in patients without IPH. McNally et al.7) performed that carotid MPRAGE in Western patients has been used in the workup of stroke in an emergent setting as our study. Carotid MPRAGE-positive signal was associated with an acute cerebral territorial ischemic event with a relative risk of 6.4. In our study, the prevalence of IPH at baseline was 30.8% and MPRAGE-positive carotid in our patients with acute neurologic symptoms had a further 3.3-fold increased risk of acute stroke on DWI compared with MPRAGE-negative carotid. This result was relatively low prevalence compared with Western study7,15). McNally et al.7) reported that a high grade carotid stenosis had a relatively increased risk of acute stroke if the lesion was MPRAGE-positive. Our study shows that MPRAGE-positive carotid in patients with carotid atherosclerotic plaque had a higher risk of acute stroke compared with MPRAGE-negative carotid.
Our study had some limitations. First, scan time in acute stroke MR examination has important implications for the time onset of treatment and prognosis. Therefore, acute stroke MR examination was generally used the limited sequences such as DWI, SWI, PWI, TOF-MRA, and contrast-enhanced MRA. Scan time of MPRAGE imaging was approximately 4 minutes, which was longer than required by other imaging methods. However, we believe that the benefits of early and accurate detection of carotid IPH awarded by this imaging technique outweigh the con of longer scan times. Second, our study was focused the atherosclerotic lesion of intracranial and extracranial vessels, although patients with intracranial atherosclerotic plaque were excluded in this study. Major causes of acute ischemic stroke are major arterial atheroma, cardioembolic sources, microvascular disease, and cryptogenic factors. Acute ischemic stroke of vulnerable carotid plaque such as IPH, fibrous cap rupture, or ulcer was relatively low prevalence compared with that of cardiac sources. Finally, our study is the lack of a gold standard histological reference. Ota et al.9) performed the diagnostic performances of three T1-weighted 3.0-T MR sequences at carotid IPH imaging, with histologic analysis as the reference standard. MPRAGE sequence, as compared with T1-weighted fast spin echo and TOF sequences, demonstrated higher diagnostic capability for the detection and quantification of IPH. Therefore, we considered that carotid MPRAGE-positive was strongly supported IPH.
CONCLUSION
MPRAGE-positive carotid plaque in population was associated with increased risk of acute territorial ischemic strokes. Especially, MPRAGE-positive in patients with carotid atherosclerotic plaque was relatively higher risk of acute ischemic stroke. This study found that IPH in carotid atherosclerotic plaque was present in a high proportion of acute ischemic strokes. Therefore, routine use of the MPRAGE sequence in stroke MR examination may lead to a more accurate stroke risk estimate and more accurate determination of a carotid source of ischemic events.
Acknowledgements
This paper was supported by Fund of Biomedical Research Institute, Chonbuk National University Hospital.