Metallothinein 1E Enhances Glioma Invasion through Modulation Matrix Metalloproteinases-2 and 9 in U87MG Mouse Brain Tumor Model
Article information
Abstract
Malignant glioma cells invading surrounding normal brain are inoperable and resistant to radio- and chemotherapy, and eventually lead to tumor regrowth. Identification of genes related to motility is important for understanding the molecular biological behavior of invasive gliomas. According to our previous studies, Metallothionein 1E (MT1E) was identified to enhance migration of human malignant glioma cells. The purpose of this study was to confirm that MT1E could modulate glioma invasion in vivo. Firstly we established 2 cell lines; MTS23, overexpressed by MT1E complementary DNA construct and pV12 as control. The expression of matrix metalloproteinases (MMP)-2, -9 and a disintegrin and metalloproteinase 17 were increased in MTS23 compared with pV12. Furthermore it was confirmed that MT1E could modulate MMPs secretion and translocation of NFkB p50 and B-cell lymphoma-3 through small interfering ribonucleic acid knocked U87MG cells. Then MTS23 and pV12 were injected into intracranial region of 5 week old male nude mouse. After 4 weeks, for brain tissues of these two groups, histological analysis, and immunohistochemical stain of MMP-2, 9 and Nestin were performed. As results, the group injected with MTS23 showed irregular margin and tumor cells infiltrating the surrounding normal brain, while that of pV12 (control) had round and clear margin. And regrowth of tumor cells in MTS23 group was observed in another site apart from tumor cell inoculation. MT1E could enhance tumor proliferation and invasion of malignant glioma through regulation of activation and expression of MMPs.
INTRODUCTION
Malignant glioma is the most malignant form of primary brain tumors and constitutes approximately 25% of primary adult brain tumors14). Despite modern diagnostics and treatments, the median survival time does not exceed 15 months. Because local invasion of malignant glioma is causative of recurrence and therapeutic failure, it is important to study the molecular basis of biological behavior of invasive tumor cells. In our previous study, we identified that Metallothionein 1E (MT1E) could modulate migration and invasion in human malignant glioma cells through in vitro experiments24).
MT1E, a small metal binding protein, is cysteine rich and has a high affinity for heavy metal ions, such as zinc, cadmium, copper, mercury, and platinum. It plays the role of metal homeostasis, heavy metal detoxification, chemoresistance, and poor prognosis of some tumors2325). A striking feature of MT1E is that they can modulate the activities of zinc dependent proteins including enzymes and zinc-finger transcription factors, by the removal and transfer of zinc18). We focused on these functions and studied nuclear factor kB (NFkB) and matrix metalloproteinases (MMPs) associated with cell migration among zinc dependent factors.
NFkB is a zinc dependent transcription factor that is involved in cellular responses to stimuli such as stress, cytokines, free radicals, ultraviolet irradiation, etc. Roles in cancer regulate various genes that control cell proliferation and cell survival5), and resistant to chemotherapeutic agents213). For NFkB activation, two main pathways exist in cells. One is the canonical pathway that is induced by most physiological NFkB stimuli and leads mainly to phosphorylation of NFkB inhibitor (IkBa) and nuclear translocation of mostly p65-containing heterodimers. Another is the noncanonical pathway that depends on IkB kinase a-mediated phosphorylation of p100 associated with p52-RelB complex and leads to partial processing of p100 and the generation of p52-RelB complexes. The released NFkB then enters the nucleus and activates the transcription of many different target genes21).
MMPs belong to a family of zinc-dependent endopeptidases that degrade the extracellular matrix. Especially, MMPs have well known effects on tumor proliferation and metastasis. They are synthesized in the latent form and secreted as proenzymes and require extracellular activation. The presence of a propeptide in the MMPs contains a cysteine residue, which forms the coordination between the cysteine thiol with the zinc atom in the catalytic domain, and prevents MMPs from being active22). Primary tumor cell invasion of the surrounding tissue is the first stage of a metastasis cascade, and many factors, such as MMPs, are associated with this stage20).
In the present study, we investigated that MT1E could actually enhance glioma invasion in vivo brain tumor model and identified the association NFkB and MMPs in glioma invasion.
MATERIALS AND METHODS
Cell culture
Human malignant glioma cell line U87MG and U343MG (Korean cell line bank, Seoul, Korea) was routinely maintained in Dulbecco's modified Eagle's medium (DMEM, GibcoBRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS, GibcoBRL, Gaithersburg, MD, USA) at 37℃ in a humidified 95% air/5% CO2 atmosphere.
siRNA oligonucleotide transfection
A small interfering RNA oligonucleotide was used for MT1E knockdown. The synthesized MT1E siRNA (5'-CCUGACUGC UUGUUCGUCU-3') and scrambled ribonucleic acid (negative control) were purchased from Bioneer (Daejeon, Korea). Approximately 2×105 cells were seeded on a plate and transfected with the siRNA oligonucleotide using Lipofectamine™ RNAiMAX (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. MT1E knockdown was confirmed by Western blotting.
Stable transfection
Preparation of metallothionein 1E construct
The complete coding region of the human MT complementary (cDNA) was amplified from U87MG cDNA by polymerase chain reaction (PCR) with synthetic primers. The polymerase chain reaction primers were designed as follows. MT-Sense : 5'-CGGGATCCATGGACCCCAACTGCTCTT-3', 5'-GCTCTAGAGCTCAGGCACAGCAG CTGCACT-3'. MT-anti sense : 5'-GCTCTAGAGCATGGACCCCAACTGCTCTT-3', 5'-CGGGATCCTCAGGCACAGCAGCTGCACT-3' The amplified cDNA was sequenced and directly subcloned into the pcDNA3.1 (+) vector (Invitrogen, San Diego, CA, USA) between the BamHI and XbaI sites, which is contained within the CMV promoter and the neomycin resistance gene. The resulting vectors were designated as pcDNA3.1-MT-S, pcDNA3.1-MT-AS.
Transfection
For ex vivo study, U343-MT-S and U87-MT-AS designated in our previous experiment were used24). In brief, U343-MT-S and U87-MT-AS were respectively transfected with sense MT1E cDNA plasmid (pcDNA3.1-MT-S) in U343MG and antisense MT1E cDNA plasmid (pcDNA3.1-MT-AS) in U87MG. And for in vivo study MTS23 cell line was newly established in U87MG as follow. The optimal cell density for transfection is normally between 50 and 80% confluency for adherent cells. Empty vector and pcDNA3.1-MT-S were respectively transfected into U87MG using Lipofectamine 2000 (Invitrogen, San Diego, CA, USA). Cells in serum-free DMEM were mixed with 1 µg of plasmid DNA and 10 µL of Lipofectamine 2000/serum-free media according to the manufacturer's protocol. After incubation at 37℃ (5% CO2) for 5 h, the transfection mixture was replaced with DMEM supplemented with 10% FBS. After 24 h incubation, the medium was replaced with DMEM containing 10% FBS and 500 ug/mL G418. The transfectants were designated as pV12 (control) and MTS23, respectively.
Preparation of total protein and conditioned media
For the preparation of total protein, cells were lysed in a protein extraction buffer [50 mM Tris (pH 8.0), 5 mM ethylenediaminetetraacetic acid, 150 mM sodium chloride, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate, 1% NP-40, 1 mM phenylmethane sulfonyl fluoride, and 1 mg/mL protease inhibitor cocktail]. For the preparation of conditioned media, cells were grown in 60-mm plates until they were subconfluent, and then 1 mL of serum-free medium was added to each plate. After incubation for 48 hr, the conditioned media were clarified by centrifugation. The protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA).
Gelatin zymography
Gelatin zymography was carried out as described previously15). Briefly, proteins (20 µg) in conditioned media were mixed with sample buffer (50 mM Tris-HCl, 2% SDS, 0.1% bromophenol blue, and 10% glycerol before electrophoresis). Aliquots were electrophoresed on 8% SDS-polyacrylamide gels containing 1 mg/mL type A gelatin (Sigma-Aldrich, St. Louis, MO, USA). Each gel was washed three times for 30 min in 2.5% Triton X-100 and then incubated for 20 h at 37℃ in incubation buffer [50 mM Tris-HCl (pH 7.5), 10 mM CaCl2, and 200 mM NaCl]. The gels were stained with Coomassie Brilliant Blue R-250 (0.2% Coomassie Brilliant Blue R-250, 20% methanol, 10% acetic acid in water) and then destained in 20% methanol and 10% acetic acid in water.
Western blot
A total of 20 µg of whole cell lysates were separated by 15% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Pall Corporation, Pensacola, FL, USA). The membrane was then incubated for 2 hrs at room temperature in TBS-T solution [10 mM Tris-Cl (pH8.0), 150 mM NaCl, and 0.05% Tween 20] supplemented with 5% non-fat dry milk and probed overnight at 4℃ with anti-MT1E (Sigma-Aldrich, Saint Louis, MD, USA), anti-MMP2, MMP9 (Abcam, Cambridge, UK), anti-Actin, NFkB p50, Lamin B and p-ikB (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-Nestin (BD Pharmingen, San Diego, CA, USA). The bound antibodies were visualized with the appropriate horseradish peroxidase-conjugated secondary antibody (Abfrontier, Seoul, Korea) conjugated with horseradish peroxidase using enhanced chemiluminescence reagents (ECL, Millipore, Billerica, MA, USA).
Brain slice invasion study of transfectants
In an attempt to recapitulate the matrix macromolecule representation normally encountered by infiltrating glioblastoma cells, we performed organotypic cultures, as previously described by Jung et al.1011). By using the DiI (1,1'-Dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate, 97%, Aldrich chem.) stained cells, it was possible to evaluate the invasiveness in a brain slice model. Briefly, rat brain was cut into 1-mm-thick, 8×8-mm2 slices using a brain matrix (Harvard instruments, Boston, MA, USA) and placed onto the upper chamber of Transwell culture dishes (0.4 µm in pore size, Coring Incorporated-Life Scences, Lowell, MA, USA). The brain slices were incubated in medium containing DMEM including 10% FBS. Five×105 tumor cells were placed on a central hole of the brain slice and incubated for 14 days.
In vivo studies
Five- to six-week old male BALB/c athymic nu-/nu- mice (body weight, 20–30 g) were purchased from the Orient Co. (Seongnam, Korea). They were housed in groups of three or four under standard conditions at a temperature of 22℃ and a 12-h light/12-h dark cycle. The mice had free access to standard food pellets and tap water. The mice were anesthetized with isoflurane (2%), and a mixture of ketamine (200 mg/kg) and xylazine (10 mg/kg). 5×105 of pV12 and MTS23 cell lines were suspended in free-DMEM and injected stereotactically into the right striatum, respectively. After 3 weeks, all mice inoculated pV12 and MTS 23 cell lines were sacrificed. Animal care, experiments, and euthanasia were performed in accordance with the protocols ap-proved by the Chonnam National University Animal Research Committee (Gwangju, Korea).
Histopathology
All mice were anesthetized and perfused transcardially with 4% zinc salt-based fixation-containing 36.7 mM ZnCl2, 27.3 mM ZnAc2·2H2O, and 0.63 mM CaAc2 in 0.1 M Tris, pH 7.4. The brain tumor was removed, fixed in the same solution for 36–38 h at room temperature, and then dehydrated for paraffin embedding. The tumors were blocked in cross-section and processed for paraffin embedding.
Three µm-thick consecutive sections were cut from the recipient blocks and placed on poly-L-lysine-coated slides for immunohistochemistry. Representative sections were stained with H&E. Heat-induced epitope retrieval was carried out for 10 min at 100℃ in a pressure cooker in 10 mM citrate buffer, pH 9.0 (DAKO, Carpinteria, CA, USA). Endogenous peroxidase activity was blocked by incubating the samples in phosphate buffer saline (PBS) containing 3% H2O2, and the non-specific binding sites were blocked with 3% bovine albumin (Sigma-Aldrich, Saint Louis, MO, USA) in PBS for 20 min at room temperature. The following primary monoclonal antibodies were added : matrix metalloproteinases-2 and MMP-9 (1 : 8000; Abcam, Cambridge, UK) and Nestin (1 : 4000, BD Biosciences PharMingen, Burlingame, CA, USA). A biotin-labeled secondary antibody (Vector Laboratory, USA) was then added, and the samples were incubated at room temperature for 1 h. A streptavidin-horseradish peroxidase detection system (Vector Laboratory, Burlingame, CA, USA) was followed by 20 min incubation at room temperature. The tissue sections were ready for the chromogen reaction with diaminobenzidine. Counterstaining was performed with Hematoxylin.
RESULTS
MT1E enhanced invasion of malignant glioma cell lines in ex vivo study
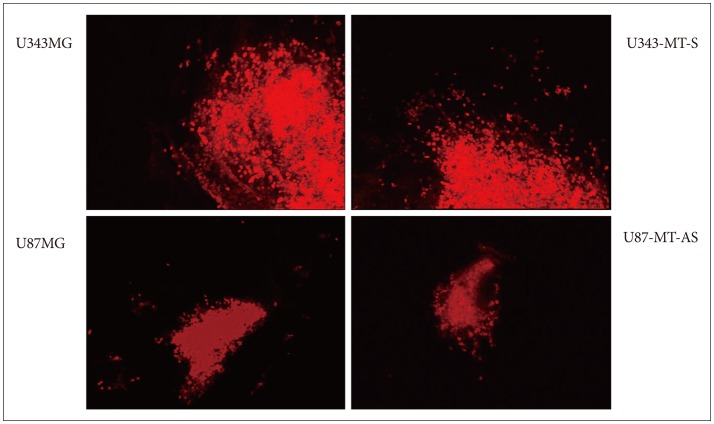
According to our previous in vitro studies, we identified MT1E could induce migration and proliferation of malignant glioma cells24). Before in vivo experiment, we desired to confirm whether MT1E could also enhanced invasion of malignant glioma through brain extracellular matrix and organotypic culture was performed1012). U343-MT-S, overexpressing MT1E, migrated widely throughout the surrounding normal brain, compared with control cells. U87-MT-AS showed less invasiveness, compared with parental cells (Fig. 1).
Endogenous MT1E content
To assess the effect of MT1E on glioma invasion according to in vivo study, 'MTS23' continuously overexpressing MT1E was established in U87MG. As shown Fig. 2A, MT1E was highly expressed in MTS23 cells, compared with pV12 (control).
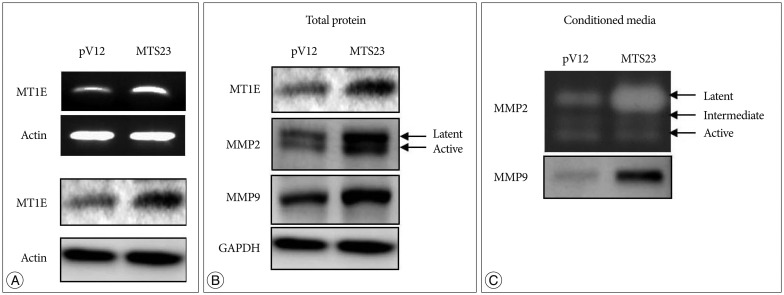
MT1E modulates MMP-2, 9 and ADAM17 in U87MG. A : The stable cell line overexpressing MT1E ('MTS23') was established and endogenous MT1E was detected by RT-PCR (upper) and Western blot (lower). 'MTS23'; U87MG cells transfected with sense MT1E plasmid, 'pV12' (control); U87MG cells transfected with empty vector. B : Western blot in cell lysate. C : Upper; Gelatin zymography, lower : Western blot in supernatant. MT1E : metallothionein 1E, MMP : metalloproteinases, ADAM : a disintegrin and metalloproteinase, GAPDH : glyceraldehyde 3-phosphoate dehydrogenase.
MT1E overexpression modulates MMP-2, 9 and ADAM17 in U87MG
On the basis of our previous study, we examined whether MT1E modulates the expression of MMP-2, 9 and a disintegrin and metalloproteinase (ADAM) 17, and the activities of MMP-2, 9. The expression of MMP-2, 9 and ADAM17 was increased in MTS23 cells overexpressing MT1E, compared with control (Fig. 2B). In general, zymography is a method for detection of MMP-2 and -9 activities, but we could not detected MMP-9 activity in U87MG according to these method. So secreted MMP-9 was detected by western blot. As results, the activity of MMP-2 and the expression of secreted MMP-9 were significantly increased in MTS23, compared with control (Fig. 2C).
For MT1E knockdown cell, NFkB p50 and B-cell lymphoma-3 (Bcl-3) expression was decreased in nuclear fraction but increased in cytosol fraction (Fig. 3A), and p-ikB expression was decreased (Fig. 3B), compared with control. The expression of MMP-1, 2, 9 and 13 in conditioned media were decreased of MT1E knockdown cell compared with control (Fig. 3C).
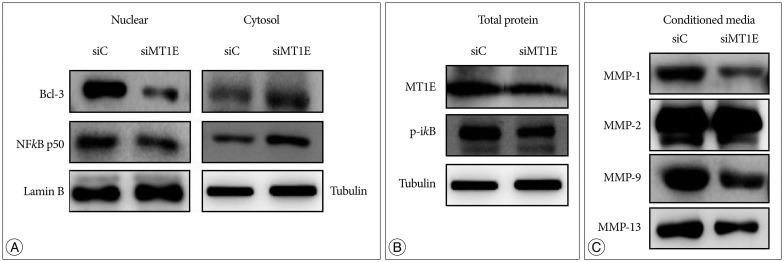
MT1E modulated secretion of MMPs through NFkB p50 and Bcl-3. The expression of these proteins was detected by Western blotting. (A) Western blot in nuclear, cytosol fraction and (B) total cell lysate. (C) Western blot in conditioned media. 'siC'; scramble RNA transfected U87MG, 'siMT1E'; siRNA for MT1E transfected U87MG. MT1E : metallothionein 1E, MMP : metalloproteinases, Bcl-3 : B-cell lymphoma-3, RNA : ribonucleic acid.
MT1E enhanced tumor invasion in mouse brain tumor models
We established brain tumor model in order to confirm whether MT1E actually modulated tumor cell invasiveness in vivo environment. Brain tumors of the group inoculating pV12 (control group) had round and clear margin (Fig. 4A), while that of MTS23 (MTS group) showed irregular margin and tumor cells infiltrating the surrounding normal brain (Fig. 4B). And regrowth of tumor cells in MTS23 group was observed in another site apart from tumor cell inoculation (Fig. 4C). Tumor volume of MTS23 group was larger than that of pV12.
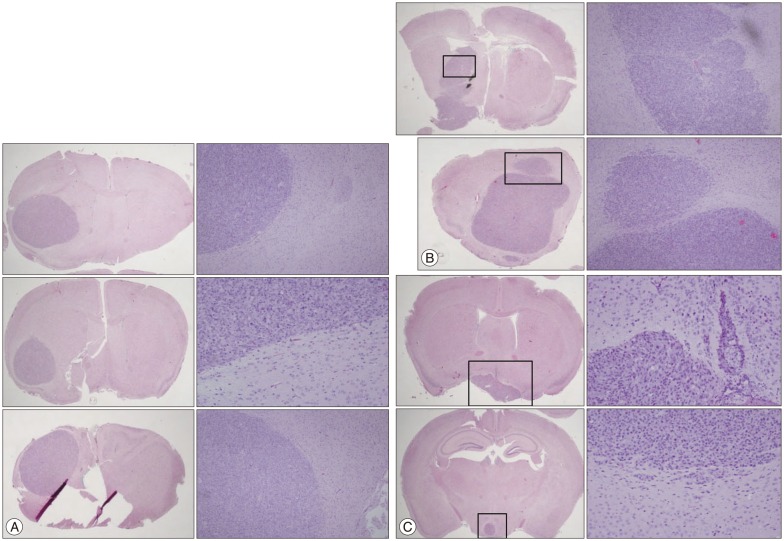
Brain tumor growth patterns of 2 groups inoculating pV12 cells and MTS23. A : pV12 implanted group. B and C : MTS23 implanted group. Tumors of MTS inoculated group showed irregular margin and tumor cells infiltrating the surrounding normal brain (B), while that of pV12 (control) had round and clear margin (A). And regrowth of tumor cells in MTS23 group was observed in another site apart from tumor cell inoculation (C) but in pV12 group was nowhere to be found.
MT1E was associated with shorter survival in mouse brain tumor models
We had detected survival rate between control group (n=10) and MTS23 group (n=10) during 70 days in order to confirm whether MT1E modulated tumor survival in vivo environment. As shown Fig. 5, the survival rate of MTS23 group was much shorter than that of pV12 control (p=0.048).
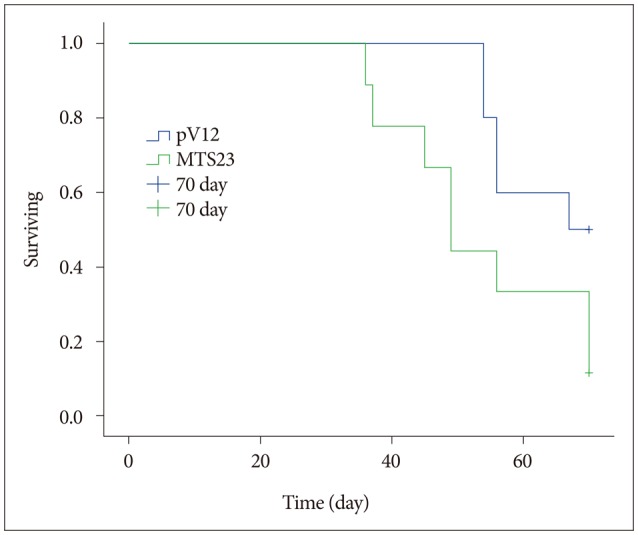
The comparison of the survival rate between 2 groups inoculating pV12 cells and MTS23. The survival rate had detected during 70 days after inoculating tumor cells and was calculated by means of Kaplan–Meier survival analysis in orthotopic mouse brain tumor models. Mice transplanted with MTS23 cells showed a longer survival than mice transplanted with pV12 cells (70 days, p<0.048).
MT1E modulated secretion of MMPs through NFkB p50 and Bcl-3
In the previous study, we mentioned the possibility of association between MT1E and MMPs and desired to confirm whether MT1E could modulate MMPs and NFkB in U87MG malignant glioma cell. In brain tumors of the MTS23 group, MMP-2 and -9 were highly expressed on marginal region and tumor cells infiltrated the normal brain, while those in the control group were expressed on tumor region. Tumor cells were identified by immunohistochemistry for Nestin (Fig. 6).
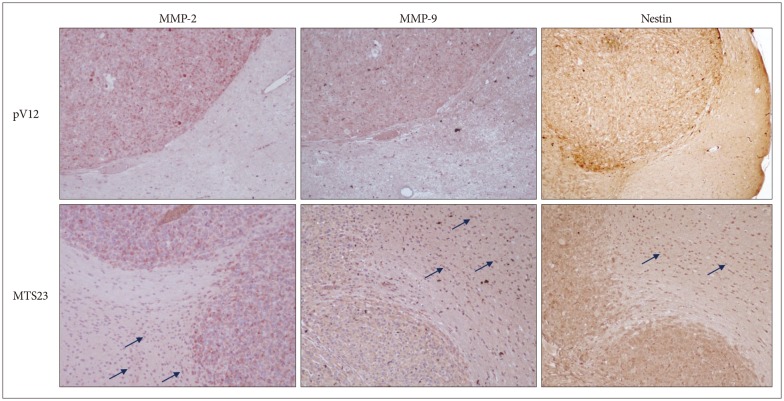
MMP-2 and -9 were highly expressed on marginal region and tumor cells infiltrated the normal brain (arrow) in MTS23 group, while those in the control group were expressed on tumor, but not on normal region. And tumor cells were identified by immunohistochemistry for Nestin. MMP : metalloproteinases.
DISCUSSION
Based on our previous study, we investigated whether MT1E actually modulates malignant glioma invasion ex vivo and in vivo. For in vivo study, MTS23 continuously overexpressing MT1E and pV12 as control were established in U87MG human malignant glioma cell line and inoculated to nude mouse brain. MTS23 group showed irregular margin and tumor cells that had infiltrated the surrounding normal brain. And tumor size was also significantly higher than the control group. Furthermore the survival rate of the mice in MTS23 group was significantly reduced, compared with the mice in the control group.
MT1E is well known to regulate essential metals such as Zn, Cu. 10% of mammalian proteasome consist of zinc binding proteins that are associated with cell signaling, gene expression, membrane structure stability, function, cell respiration, etc8). These functions of MT1E may be associated with rapid tumor growth. There are some reports that MT is related to tumor grade, malignancy, and prognosis in certain tumors such as colon cancer3), lung cancer26), brain tumors etc7), and breast cancer9). Zinc is also essential for endopeptidase proteolytic capacity to degrade the extracellular matrix, compounds with zinc-chelating groups8). We focused on these functions of MT1E, and identified whether MT1E could regulate the expression of NFkB p50 and Bcl-3 transcription factors and MMPs for enhancing glioma invasion through in vitro and in vivo studies.
MMPs are a family of Zn2+-dependent endopeptidases that are capable of degrading the components of extracellular matrix. They have been regarded as critical factors promoting tumor cells invasion. The catalytic domain of all MMPs contain a Zn2+ ion coordinated by a tris (histidine) motif; the Zn2+ ion is critical for both substrate binding and cleavage1). This Cys-Zn2+ coordination keeps proMMPs inactive by preventing a water molecule essential for catalysis from binding to the zinc atom. The enzymes are activated when a prosegment peptide is pulled away from the active center by breaking the cysteine-zinc contact and cleaved from the proenzyme. The zinc binding motif and the Met-turn are also conserved in members of the ADAM family1619). Among them, ADAM17 has been reported to be associated with migration and invasion of various tumor cell such as glioblastoma4), non-small cell lung cancer17) and breast cancer6).
MMPs has proteolytic activity after secretion in extracellular matrix. So it examined the change of the expression level of MMPs in both total cell lysates and conditioned media. As expected, the expression of MMP-1, 2, 9 and 13 secreted in conditioned media were decreased in transiently MT1E knockdown cells, compared with control. Reversely, in MTS23 cells continuously overexpressing MT1E, the expression of MMP-2, 9 and ADAM17 was increased and the activation of MMP-2 and 9 in conditioned media were significantly increased, compared with pV12 cells (control). Furthermore, MMP-2 and 9 were highly expressed in cells infiltrated the normal brain of group inoculating MTS cells in vivo.
NFkB, also a zinc-dependent transcription factor, plays a piv-otal role in a diverse array of cellular activities and gene activations (30). There are strong pathophysiologic functional similarities between MT and NFkB. Our previous study showed that MT1E regulated NFkB p50 and Bcl-3 transcription factors, and this was confirmed in U87MG knock-downed MT1E using siRNA technique. The expression of p-ikB was decreased and translocation of Bcl-3 and NFkB p50 from cytoplasm to nuclear was also decreased compared with the control cells.
CONCLUSION
In the present study, we confirmed that MT1E could enhance the invasion and proliferation of malignant glioma cell and induced expression of MMP-2 and 9 in mouse brain tumor model. And MMPs secretion and NFkB/p50 expression were also changed by MT1E. Despite the fact that the direct relation between MMPs and NFkB/p50 was not identified, on the basis of our results and reports, we may be able to present two mechanisms. One is that MT1E may regulate MMPs activation by regulating Zn contained in MMPs structure, such as a Zn donor or recipient. The other is that MT1E may induce the activity of NFkB p50/Bcl-3, following MMPs activities in malignant glioma cell lines. Finally, this series of events enhances the invasion of malignant glioma.
Acknowledgements
This study was supported by a grant (HCRI15008-1) Chonnam National University Hospital Biomedical Research Institute.