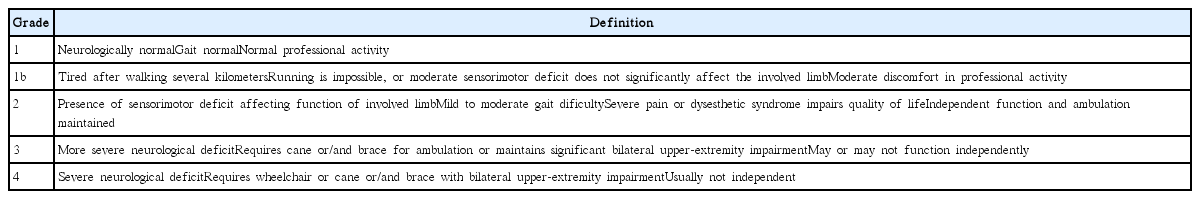
Spinal Cord Subependymoma Surgery : A Multi-Institutional Experience
Article information
Abstract
Objective
A spinal cord subependymoma is an uncommon, indolent, benign spinal cord tumor. It is radiologically similar to a spinal cord ependymoma, but surgical findings and outcomes differ. Gross total resection of the tumor is not always feasible. The present study was done to determine the clinical, radiological and pathological characteristics of spinal cord subependymomas.
Methods
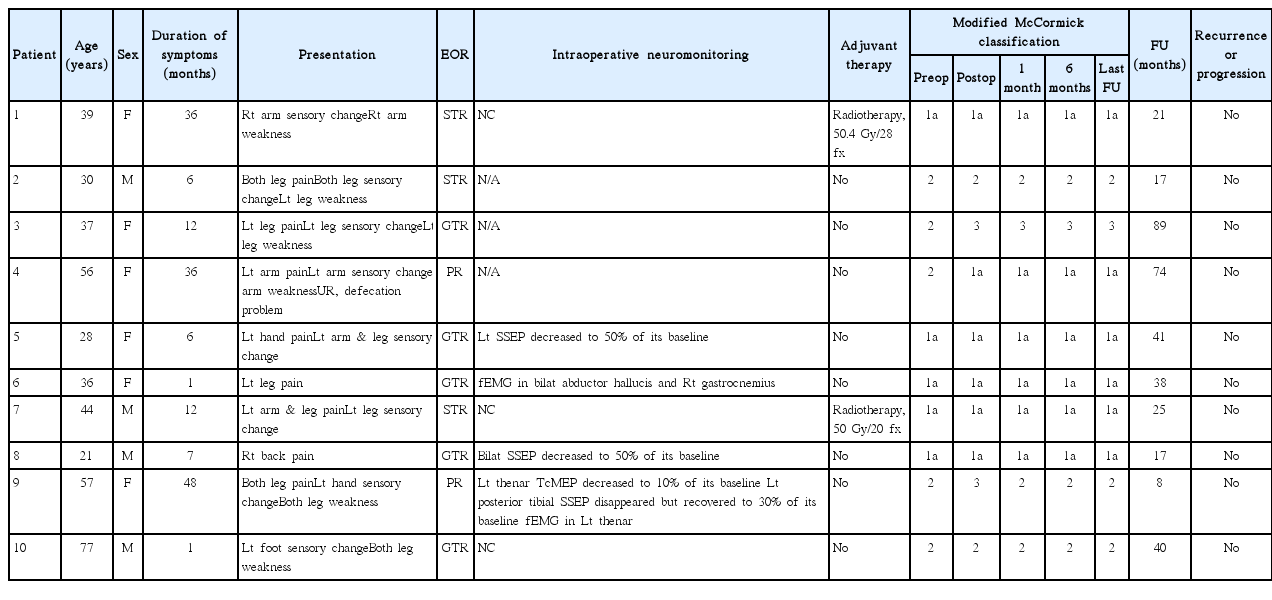
We retrospectively reviewed the medical records of ten spinal cord subependymoma patients (M : F=4 : 6; median 38 years; range, 21–77) from four institutions.
Results
The most common symptoms were sensory changes and/or pain in eight patients, followed by motor weakness in six. The median duration of symptoms was 9.5 months. Preoperative radiological diagnosis was ependymoma in seven and astrocytoma in three. The tumors were located eccentrically in six and were not enhanced in six. Gross total resection of the tumor was achieved in five patients, whereas subtotal or partial resection was inevitable in the other five patients due to a poor dissection plane. Adjuvant radiotherapy was performed in two patients. Neurological deterioration occurred in two patients; transient weakness in one after subtotal resection and permanent weakness after gross total resection in the other. Recurrence or regrowth of the tumor was not observed during the median 31.5 months follow-up period (range, 8–89).
Conclusion
Spinal cord subependymoma should be considered when the tumor is located eccentrically and is not dissected easily from the spinal cord. Considering the rather indolent nature of spinal cord subependymomas, subtotal removal without the risk of neurological deficit is another option.
INTRODUCTION
A spinal cord subependymoma (SCSE) is a benign, non-invasive, slow-growing, World Health Organization (WHO) grade I spinal cord tumor16), first reported by Boykin et al.6) in 1954. Since then, 72 cases of SCSE have been reported1–3,5–15,17–21,24–27,29,30,32–41,43–49). Although SCSE is not rare, it is not easily differentiable from a spinal cord ependymoma with radiological findings. However, surgical findings and outcomes differ from those of an ependymoma, including a high risk of neurological deficit in the event of a poor dissection plane from the spinal cord with a low rate of recurrence. We reviewed the clinical, radiological and pathological characteristics of ten cases of SCSE from the Korea Spinal Oncology Research Group (KSORG) database.
MATERIALS AND METHODS
The KSORG was established in 2009 by spine surgeons from the Seoul National University Hospital, Seoul National University Bundang Hospital, Samsung Medical Center, and several affiliated hospitals sought to promote clinical research on the management of spinal and spinal cord tumors and to develop educational programs to improve neuro-oncological care23,28,42). The principal goal of the KSORG was to evaluate surgical treatments of spinal neoplasms in a prospective and retrospective multicenter clinical series. From April of 2000 to September of 2014, ten patients in total were pathologically confirmed as SCSE patients in four hospitals. During the same period, 88 cases of spinal cord ependymoma were surgically treated28). These medical records, including radiological images, were retrospectively reviewed.
The characteristics and duration of symptoms, preoperative neurological function, intraoperative surgical findings, the extent of surgical resection, intraoperative electrophysiologic monitoring results, the use of an adjuvant treatment, the follow-up period, and postoperative complications were reviewed. The modified McCormick classification (MMC) (Table 1)31) was applied to the assess neurological functions of the patients before surgery, at discharge, at one and six months after surgery and annually thereafter. Magnetic resonance (MR) images were taken in all patients before and after surgery. The location and level of involvement of the tumor and the pattern of enhancement were evaluated in detail. In our study, all tumors were removed with a conventional posterior approach by senior spine tumor surgeons, and microsurgical techniques did not differ from previously published techniques in the literature.
The extent of resection was classified as gross total resection (GTR), subtotal resection (STR) or partial resection (PR). GTR was defined if surgeons described a complete resection of the tumor and there was no evidence of the tumor in postoperative MR images. If a small piece of tumor was left in place according to the surgeon’s decision or an obvious retained fragment appeared in postoperative MR images (80–99% resection), the extent of resection was considered to be STR. In the same manner, cases involving less than 80% resection were defined as PR. Pathologically, after resection of the tumor, experienced neuropathologists in each institution reviewed both frozen sections and permanent specimens. The WHO classification system was used for histopathological diagnoses. Immunohistochemistry and electron microscopic examinations were performed as needed.
Patient follow-up took place at an outpatient clinic postoperatively to evaluate their neurological statuses with MMC grades. Tumor recurrence and progression were defined both clinically and radiologically. Tumor recurrence was defined as regrowth of the tumor after GTR on follow-up MR images or any clinical aggravation. Tumor progression was defined as tumor regrowth after STR or PR on follow-up MR images or any clinical aggravation. The study was reviewed and approved by the institutional review board (IRB No. 1306-106-500).
RESULTS
Patient demographics
Table 2 summarizes the characteristics of all patients. Of the ten, four were male and six were female, with ages at surgery ranging from 21 to 77 years (median, 38 years). The most common symptoms were sensory changes and pain in eight patients, followed by motor weakness in six, and bowel/bladder symptoms in one patient. The median duration of symptoms was 9.5 months (range, 1 to 48). The ratio of SCSE versus spinal cord ependymoma was 1 : 8.8 with the present database23,28,42).
Radiological findings
Table 3 summarizes the radiological findings of all patients. The tumors were located at the cervical spinal cord in four patients, the thoracic spinal cord in four patients, and the thoracolumbar spinal cord in two. MR images of all patients showed iso- to low signal intensity on T1-weighted images (WI) and high signal intensity on T2–WI (Fig. 1). The pattern of contrast (gadolinium) enhancement was homogeneously strong in one patient, with partial uptake in three patients and scanty enhancement in six patients. The tumors were located eccentrically in six patients. Syringomyelia existed in two patients, but intratumoral cystic changes, a hemosiderin cap, or calcification was not observed. The preoperative radiological diagnosis was ependymoma in seven patients and low-grade glioma in three patients, but a subependymoma was not included in the list of differential diagnoses.

Preoperative magnetic resonance (MR) images showing an intramedullary mass located at the C2–7 level with associated hydrosyrinx extending to T3 level. A : Sagittal T1-weighted image without contrast showing isointense lesion. B : Sagittal T1-weighted image with contrast showing poor gadolinium enhancement. C : Sagittal T2-weighted image exhibiting hyperintense lesion. D : Axial T2-weighted image showing the mass located at left and dorsal side of the spinal cord. E : Postoperative T2-weighted image revealing partial removal state of the tumor.
Surgical findings
All operations were performed with the conventional posterior midline approach with laminectomy in a prone position. Tumors were grayish in color with a lobulated contour and mild vascularity (Fig. 2). All tumors were intramedullary located, and additional exophytic growth was observed in one case. In five patients, tumors showed a well-demarcated margin from the spinal cord, and GTR was achieved. In contrast, in the others, the dissection plane was not well developed, with STR performed in three patients and PR in two. Multimodal intraoperative neuro-monitoring (INM) with the transcranial motor-evoked potential (TcMEP), somatosensory evoked potential (SSEP) and free-running electromyography (fEMG) was performed in seven patients22). Three patients showed a decrease of TcMEP or SSEP and two patients revealed fEMG events during tumor dissection (Table 2).
Clinical outcome
Neurological deterioration occurred in two patients after surgery. In case 9, the MMC grade was 3 after PR and returned to its preoperative state (MMC grade 2) one month after surgery. In case 3, the MMC grade was 3 after GTR without recovery to the preoperative state (MMC grade 2). No patient underwent a second surgery due to recurrence or progression of the residual tumor, and revision surgery was performed in one patient due to a surgical site infection. Adjuvant radiotherapy was performed for two patients with STR. During the median of 31.5 months (range, 8 to 89) of follow-up, recurrence or progression of the tumor was not observed (Table 2).
Pathological findings
Frozen sectioned examination was performed in nine out of ten patients. Interestingly, an intraoperative histologic diagnosis was made correctly in only one patient, whereas eight patients were misdiagnosed as a low-grade glioma. Histopathologically, these tumors were characterized by a lobular architecture and clusters of tumor cells, which resulted in an alternating cellular and acellular pattern (Fig. 3A and B). There were no high-grade features. Mitoses were scarce and necrosis was not present. However, a degenerative nuclear pleomorphism was found in the focal area (Fig. 3C). The vasculature was often prominent and perivascular hyalinization was noted. Immunohistochemically, glial fibrillary acidic protein (GFAP) was diffusely and robustly positive in the tumor cells (Fig. 3D), but IDH-1 and Olig2 were negative. The Ki-67 labeling index was less than 1% (Fig. 3E). An electron microscopic examination was performed in three patients. Ultrathin sections showed loosely arranged round to oval tumor cells. The cells had some cytoplasmic processes filled with glial-type intermediate filaments. The perikaryal cytoplasm contained mitochondria and ribosomes. Well-developed intracytoplasmic lumina with microvilli and cilli ranged from numerous to rare.
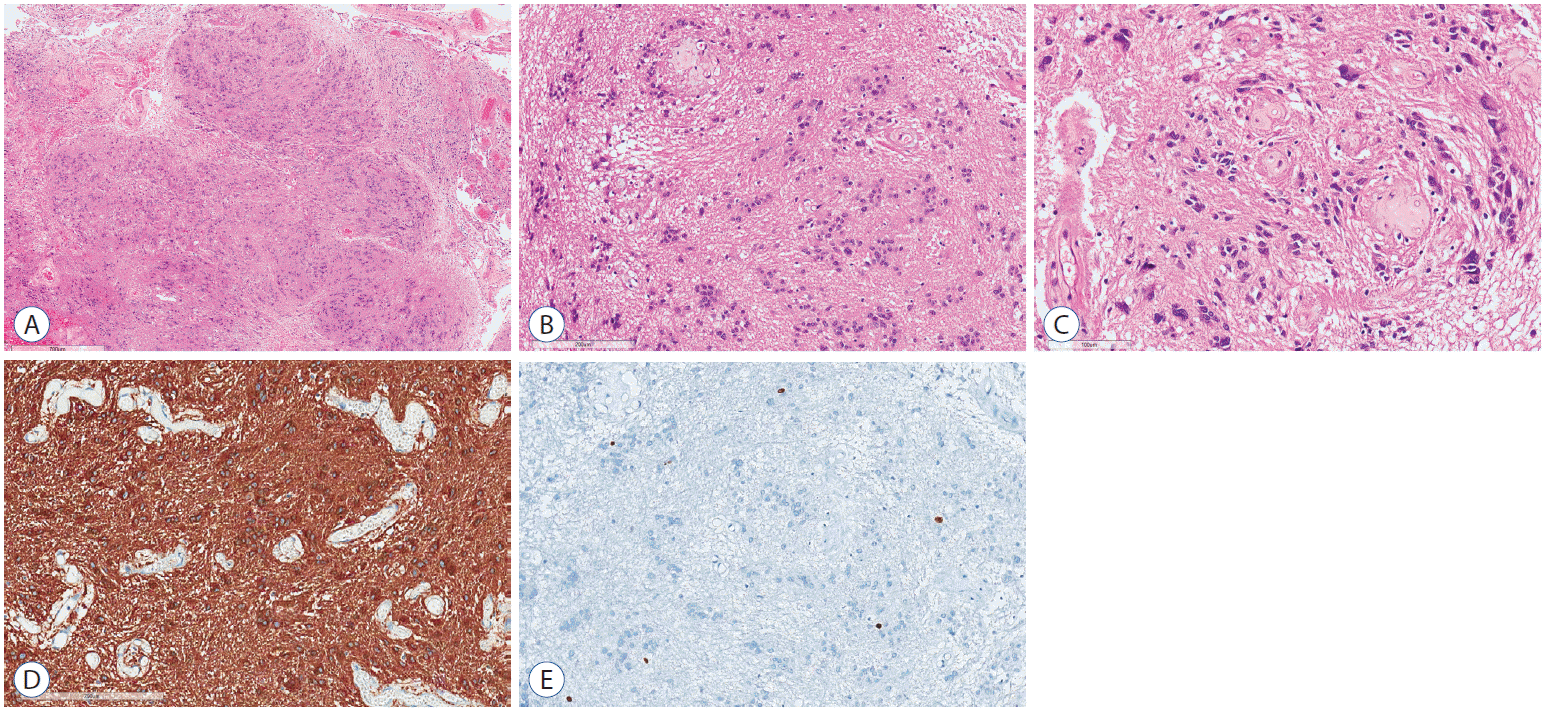
A : Hematoxylin and eosin (H&E) staining of resected specimens showing lobular architecture (H&E, ×20). B : Clustered nuclei (H&E, ×100). C : With focal degenerative nuclear enlargement or pleomorphism (H&E, ×200). D : Immunostaining of specimens showing diffuse positive to GFAP (GFAP immunostainig, ×150). E : Ki-67 index was less than 1% (Ki67, ×150). GFAP : glial fibrillary acidic protein.
Illustrative case
Case 9
A 56-year-old woman presented a four-year history of progressive bilateral leg numbness. She also revealed hypesthesia at the fourth and fifth fingers of her left hand, lasting for a year. Neurological examination revealed motor grade 4+/5 weakness in both lower extremities, which resulted in a mild gait disturbance. MR images showed an eccentrically located mass, producing a hyper-intense signal in T2-weighted and an iso-intense signal in T1-weighted images with focal weak gadolinium enhancement. The mass was extended to the left dorsal surface, ranging from the C3 to C7 level, and syringomyelia was combined below the mass (Fig. 1).
The patient underwent a C3–C7 laminectomy, midline durotomy and arachnoid incision. The exposed spinal cord was rotated to the right side (Fig. 2A). Midline myelotomy was performed and a gelatinous grayish tumor was exposed (Fig. 2B). The intraoperative histological diagnosis in this case was a low-grade glioma. The tumor was firm and strongly adherent to the normal spinal cord parenchyma, making dissection from the spinal cord difficult. During the dissection, INM revealed repeated abnormal fEMG at left thenar muscle and a reduction of the left thenar TcMEP amplitude to 10% of its baseline voltage. The left posterior tibial SSEP also disappeared. Thus, we stopped further dissection and the tumor was partially removed with a cavitron ultrasonic aspirator. The left posterior tibial SSEP amplitude recovered to 30% of its baseline voltage at the end of the surgery; however, the left thenar MEP amplitude did not recover.
A histopathological examination revealed typical findings of a subependymoma. A lobular architecture and clusters of tumor cells with an alternating cellular and acellular pattern were found without mitoses or necrosis. Immunohistochemically, GFAP was diffusely positive and the Ki-67 labeling index was less than 1% (Fig. 3). Postoperatively, the patient presented grade 3/5 weakness and hypesthesia of the bilateral lower limbs and left thenar muscle. At the postoperative one-month follow-up assessment, the patient recovered to her preoperative neurological status. She was followed up for eight months without evidence of regrowth.
DISCUSSION
Since the first report by Boykin et al.6) in 1954, 72 cases of SCSE in total were reported in the literature before this study1–3,5–15,17–21,24–27,29,30,32–41,43–49). Most of the studies were case reports, and the largest study was by Wu et al.47) involving 13 cases from a single institution. Table 4 summarizes the SCSE characteristics in the previously reported cases and in the current study5,6,10,12,17–21,25,44,45,47–49). Because a SCSE is a benign and indolent WHO grade I tumor, GTR is not always necessary. In contrast, a spinal cord ependymoma is a WHO grade II tumor, and the extent of resection significantly affects the progression-free survival rate28). Thus, a preoperative differential diagnosis of SCSE especially from a spinal cord ependymoma is important when devising an optimal surgical plan. However, a preoperative differential diagnosis remains difficult due to the lack of specific clinical and imaging characteristics.
Preoperative clinical and radiological findings
In this study, the most common symptoms were sensory changes and pain followed by motor weakness and bladder symptoms. In the literature, instances of SCSE were most commonly located at the cervical spinal cord, followed by the thoracic spinal cord and the lumbar spinal cord (Table 4). In this study, SCSEs were most commonly located at the cervical and thoracic cord followed by the thoracolumbar cord. The location of the tumor was not discernable from cases of spinal cord ependymoma28).
Common MR imaging findings of SCSEs were iso- to low signal intensity on T1–WI and high signal intensity on T2–WI, and these features were not different from those associated with a spinal cord ependymoma. However, the eccentric location, poor gadolinium enhancement, and rare intra-tumoral cysts and calcification may characterize SCSEs.
In the present study, the eccentric location in the axial MR images can serve as a clue for distinguishing a SCSE from an ependymoma, which was observed in five out of ten cases. In previous reports, 86% of cases (43 cases/50 cases) showed an eccentric location. However, this finding may be indistinct if the tumor is large enough to occupy the entire spinal cord in the axial view of MR images, as shown in Fig. 1. Eccentricity may be a clue only when the tumor is not large enough to occupy the entire spinal canal.
In this study, only one patient showed homogeneous strong contrast enhancement in the tumor, and such an enhancement was observed only in 24.3% of patients in previous reports. Although syringomyelia was combined in two patients in the present study, an intratumoral cyst or calcification was not found, in accordance with previous reports1–3,5–15,17–21,24–27,29,30,32–41,43–49).
Intraoperative findings and surgical outcomes
Although preoperative radiological findings were similar, intraoperative findings were not similar between cases of spinal cord ependymoma and SCSE. During surgery, the interface between the tumor and the spinal cord was clear only in half of the patients, whereas in the others, the dissection plane was not clearly developed. The tumors were completely removed only in 50% of cases, despite the fact that all surgical procedures were performed by senior spine surgeons who have more than ten years of experience with spinal cord tumors. The rate of GTR was much lower than the GTR rate of spinal cord ependymomas (72/88, 81.8%) during the same period by the same surgeons28). In the literature, the GTR rate of SCSEs was 73.9%, which is higher than that in the present study but nonetheless lower than the GTR rate of spinal cord ependymomas. There are two possible reasons for this : the poor dissection plane between the tumor and the spinal cord and the inaccurate intraoperative histological diagnosis as a low-grade glioma.
There was no recurred case in the present study during the median 31.5 months follow-up period (range, 8–89). Before this study, there have been three reported cases of recurrence after surgical resection of the SCSEs3,15,40). Follow-up periods of recurred cases were 7, 9, and 12 years, which is longer than that of all reported SCSEs (mean 45.7 months, median 39 months)1–3,5–15,17–21,24–27,29,30,32–41,43–49). This result showed that long term follow-up is essential, though recurrence of SCSE is rare.
Pathological findings
The histopathogenesis of a subependymoma has yet to be revealed clearly. An intracranial subependymoma is currently thought to derive from subependymal glial precursor cells, which are bipotential cells with the ability to differentiate into either ependymal cells or an astrocyte4). On the other hand, in SCSE, Krishnan et al.25) suggested subpial spinal white matter progenitor cells as a possible histogenesis for a better explanation of the predominant peripheral and exophytic location. This may explain the eccentric location of a SCSE. Histological features are distinct from spinal cord ependymomas and spinal cord astrocytomas.
However, differentiation with an intraoperative frozen section approach was challenging. In this study, intraoperative histologic diagnoses by frozen section examination were performed in nine out of ten patients; however, eight patients were diagnosed as having a low-grade glioma, and a correct diagnosis was made in only one case. Given that a subependymoma has both ependymal and glial components histologically, this high rate of misdiagnosis was attributed to the small number of specimens and a lack of suspicion. This problem can be overcome by sending a sufficient amount of specimens to a pathologist with appropriate suspicion by the clinician.
Treatment recommendation
Recommended treatments for SCSEs vary depending on the author. Matsumoto and Nakagaki29) and Jallo et al.20) insisted that GTR should be conducted for a better clinical outcome, though GTR may not be possible in all cases. Wu et al.47) and Jang et al.21) showed that STR or PR was sufficient for a good clinical outcome when GTR is not feasible.
Because SCSEs are benign tumors and given that tumor progression is rare after STR or PR47), impellent GTR in the face of neurological damage cannot be recommended. The preservation of neurological function and the quality of life should be considered as the most crucial points. Thus, we propose STR without the risk of neurological deficit, if GTR is not feasible. Although adjuvant radiotherapy was performed in two patients after STR in the present study, it is not usually recommended.
CONCLUSION
A spinal cord subependymoma is a benign and indolent tumor. Clinical and radiological features are similar to those of an ependymoma, but a spinal cord subependymoma needs to be considered when the tumor is located eccentrically and not easily dissected from the spinal cord. Impellent gross total removal may result in a neurological deficit. Considering the benign nature of subependymomas, subtotal removal without the risk of any neurological deficit may be a viable alternative option.
Limitation
Because SCSEs are uncommon spinal cord tumors, retrospective data from multiple institutes were utilized, but the small number of patients was the major limitation here. In the present study, although experienced specialists were involved in the radiological and pathological (intraoperative and permanent) diagnosis and surgeries, differences in experience must be considered. Most importantly, the follow-up period was not long enough to provide a treatment strategy. Nonetheless, we showed the importance of the preoperative suspicion of a SCSE and suggested STR or PR as an alternative option. Apparently, SCSEs are similar to spinal cord ependymomas, but the treatment strategy may differ, and this needs to be included in any differential diagnosis.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2014R1A2A1A11049662), and by the NRF grant funded by the Korea government (MSIP) (No. 2010-0028631).
Notes
CONFLICTS OF INTEREST
The authors have no financial conflicts of interest.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in this study.