Transarterial Embolization of Intracranial Arteriovenous Fistulas with Large Venous Pouches in the Form of Venous Outlet Ectasia and Large Venous Varix or Aneurysm : Two Centers Experience
Article information
Abstract
Objective
There are different types of cerebral vascular malformations. Pial arteriovenous fistulas (PAVFs) and dural arteriovenous fistulas (DAVFs) are two entities; they consist of one or more arterial connections to a single venous outlet without a true intervening nidus. The high turbulent flow of PAVFs and aggressive DAVFs with cortical venous reflux can result in venous outflow varix and aneurysmal dilatation. They pose a significant challenge to transvenous embolization (TVE), stereotactic radiosurgery, and surgical treatment. We aim to share our centers’ experience with the transarterial embolization (TAE) for arteriovenous fistulas (AVFs) with large venous pouches and to report the outcome.
Methods
The authors’ two institutions’ databases were retrospectively reviewed from February 2017 to February 2021. All patients with intracranial high flow PAVFs and aggressive DAVFs with venous outlet ectasia and large venous varix and were treated by TAE were included.
Results
Fifteen patients harboring 11 DAVFs and four PAVFs met our inclusion criteria. All patients underwent TAE in 17 sessions. Complete angiographic obliteration was achieved after 14 sessions in 12 patients (80%). Four patients (25%) had residual after one TAE session. Technical failure was documented in one patient (6.7%). Fourteen patients (93.3%) had favorable functional outcome (modified Rankin score 0–2).
Conclusions
TAE for high flow or aggressive intracranial AVFs is a safe and considerable treatment option, especially for those associated with large venous pouches that are challenging and relatively high-risk for TVE.
INTRODUCTION
There are different types of cerebral vascular malformations. Pial arteriovenous fistulas (PAVFs) are rare entities where one or more pial arteries communicate directly with a cortical vein, accounting for 1.6% of all vascular lesions [9,11,17]. Dural arteriovenous fistulas (DAVFs) are abnormal connections between dural venous sinuses and dural or pachymeningeal branches of the internal carotid, external carotid, and vertebral arteries account for 10–15% of all intracranial arteriovenous malformations. Both PAVFs and DAVFs are distinct from the more common cerebral arteriovenous malformations; they consist of one or more arterial connections to a single venous outlet without a true intervening nidus [16-18].
The high turbulent flow of PAVFs can result in venous outflow varix and aneurysmal dilatation [10,12,26]. The same sequence can occur with aggressive DAVFs (Borden type III, Cognard type IIb-V) with cortical venous reflux. Both can lead to subsequent cerebral venous hypertension and hemorrhage [4,6,8]. Treatment for such type of fistulas is inevitable and includes endovascular treatment, surgery, or stereotactic radiosurgery (SRS). The transvenous approach has been significantly considered by neurointerventionists in the past few years, especially in fistulas with multiple small feeders [12-15]. However, the high flow arteriovenous fistulas (AVFs) with venous outflow dilatation, varix, and even aneurysms pose a significant challenge to transvenous embolization (TVE), SRS, and surgical treatment given to the fragility and long length of the venous access, and its deep location in some cases [5,7,9,11-13,21,28].
Our study aims to share our centers’ experience with the transarterial approach in embolization of PAVFs and DAVFs associated with venous outlet ectasia and reporting the outcome.
MATERIALS AND METHODS
After Institutional Review Board approval was obtained (with code number : R.21.02.1228 and 161), the authors’ two institutions’ databases were retrospectively reviewed for intracranial arteriovenous fistulas from February 2017 to February 2021. Informed written consent was exempted, as it is a retrospective study.
Inclusion criteria
All of included intracranial AVFs were considered challenging to other treatment options as surgery, SRS or TVE given to the fragility and long length of the venous access, and its deep location in some cases. We did include all patients with intracranial high flow PAVFs and aggressive DAVFs that were associated with venous outlet ectasia and large venous varix or aneurysm accompanied with or without venous hypertension and were treated with endovascular transarterial embolization (TAE) in the study. Clinical presentation, location, type, agent and techniques used, complications, and clinical and radiological follow-up were included.
Exclusion criteria
We did exclude patients with direct carotid-cavernous fistulas, galenic venous malformations, and dural arteriovenous malformations in neonates. We consider those patients diagnosed with special pathological entities and have well-recognized and established therapeutic protocols [3,19,24].
RESULTS
Patient’s characteristics
Fifteen patients harboring 11 (Cognard type IIb-V) DAVFs and four (single hole) PAVFs met our inclusion criteria. Seven were females, and eight males. Their ages ranged from 4.5 to 65 years old (mean, 34.7 years). Headache (87%) and tinnitus (40%) were the most prevalent presentations, while six patients (40%) developed intracranial hemorrhage (ICH). Only one patient required the surgical evacuation of ICH. None of these patients had prior treatment for the AVF (Tables 1 and 2).
Technical aspects of TAE
All patients underwent TAE using the EVOH copolymer (Onyx-18, Medtronic, Minneapolis, MN, USA; or Squid-12, Embof lu, Gland, Switzerland) or (n-butyl cyanoacrylate [NBCA] glue mixture with tantalum) in 17 sessions. It was accomplished in one session in 10 patients (66.7%) and two sessions in two patients (13.3%). Arterial inflow control during TAE was sought in five patients (33.3%) (Table 3).
Outcomes
Complete angiographic obliteration was achieved after 14 sessions in 12 patients (80%) as two of the patients underwent another TAE session. Technical failure was documented in one patient (6.67%) and underwent another TAE session. Four patients (25%) showed residual on angiography after one TAE session; one had another session of TAE and three patients are planned to have further treatment with TVE. Technical complication (Alopecia errata) occurred in only one patient (6.7%). Fourteen patients (93.3%) had favorable functional outcome according to the modified Rankin score (mRS; 0–2). One patient had improved after TAE from mRS 4 to 3. Follow-up duration (1 to 36 months) (Table 4).
Case illustration
Case 1
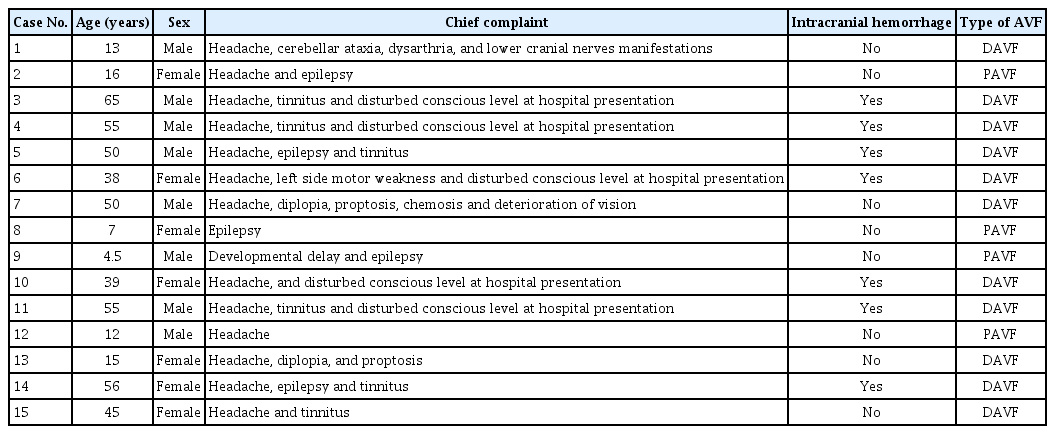
Male aged 13 years old manifested by disabling cerebellar and progressive venous hypertension signs. A digital subtraction angiogram (DSA) revealed a Congard type IV DAVF at the left transverse sinus. It was supplied by : ipsilateral middle meningeal artery (MMA) and occipital artery (OA), posterior auricular artery, artery of Bernasconi and Cassenari, and pachymeningeal branches of the posterior cerebral artery (PCA). It drained into the ectatic transverse on both sides, and right sigmoid sinuses and internal jugular vein. The left sigmoid sinus was thrombosed and occluded. Cortical venous reflux was evident too (Fig. 1). The patient underwent one session of TAE with onyx for pachymeningeal branches of PCA, and meningeal branches of MMA and OA. A detachable Apollo 3 cm microcatheter was advanced over Mirage 0.08 microguidewire just proximal to the fistulous connections supplied from PCA, and onyx was injected. Control angiogram revealed no more filling of the fistula on the arterial phase. Afterward, a Septer C balloon microcatheter was advanced to select fistulous supply from the MMA and OA and subsequent onyx injection under arterial inflow control after balloon inflation.
Case 1. A : Left vertebral artery angiogram (anteroposterior view) demonstrating Cognard type IV DAVF, supplied by pachymeningeal of posterior cerebral artery with early-dilated venous pouch (Transverse sinus). B : Left external carotid angiogram (lateral view) demonstrating Cognard type IV DAVF, supplied by meningeal branches of middle meningeal, posterior auricular and occipital arteries with early-dilated venous pouch (Transverse sinus). C : Magnetic resonance venography (showing hypertrophied dural venous sinuses and thrombosed left sigmoid sinus. DAVF : dural arteriovenous fistula.
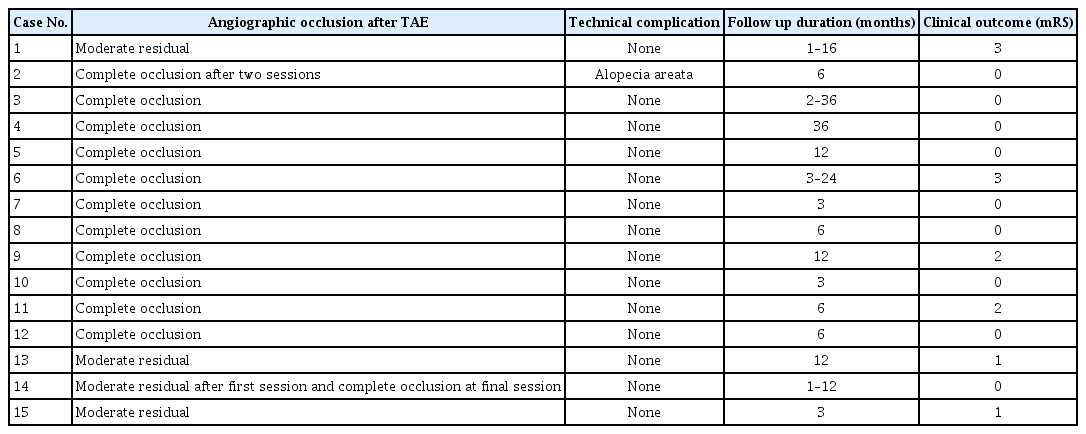
Given that there was no more arterial feeders suitable to be engaged with microguidewire/catheter system, a final angiogram was done and revealed a progressive reduction and occlusion of the arteriovenous shunting and some residual filling from other small arterial supplies. Post-procedural, the patient showed significant clinical improvement regarding his cerebellar signs and better neurological functional outcome (mRS 4 to 3). Radiological follow-up after 6 months and 1 year revealed the same residual shunting, and further treatment with TVE is being considered to achieve a complete cure later on (Fig. 2).
Case 1. A : Final angiogram showing complete occlusion of the DAVF with onyx (white arrow). B : Fluoroscopic unsubtracted image showing the Onyx cast at the fistula point. C : Road map image showing placement of Scepter C balloon catheter in the middle meningeal artery (white arrow). D : Three-dimensional reconstructed angiographic done after 1 year, showing residual filling of DAVF. DAVF : dural arteriovenous fistula.
Case 2
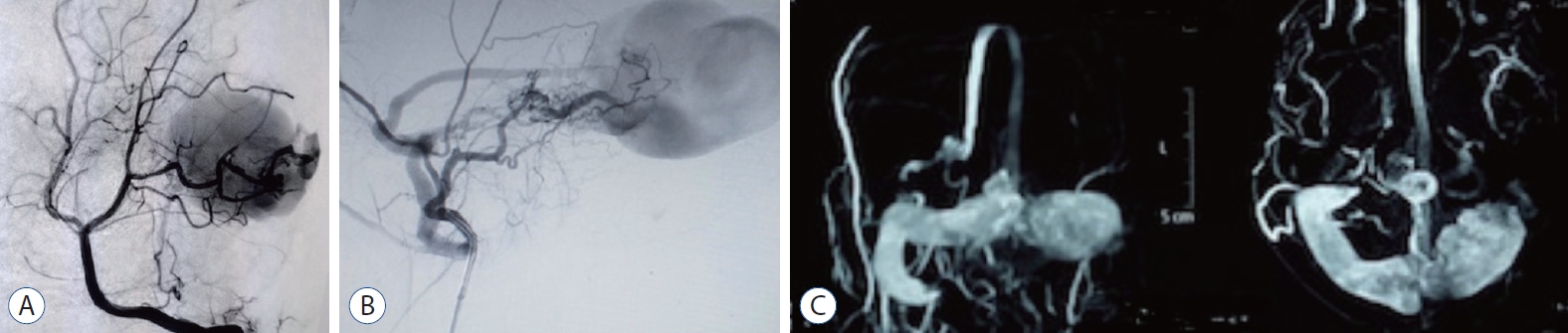
Female aged 16 years old manifesting headache and absence fits for 6 months period. A DSA revealed a left occipital high flow PAVF between left hypertrophied PCA (P3 segment) and occipital vein. The draining vein shows aneurysmal dilation measuring 5 cm in its widest dimension, with subsequent ectasia of the draining sinuses. The decision was taken to use a double microcatheter interlocking coil embolization technique. Two excelsior SL 10 catheters were navigated to the fistulous connection. A subsequent synchronous delivery of two Axium 3D coils from the two catheters was tried many times using variable sized coils. However, the high turbulent flow did not help coils stabilization or their interlocking. The control angiogram, in turn, did not show any significant reduction of the arteriovenous shunting, and the session was aborted (Fig. 3).
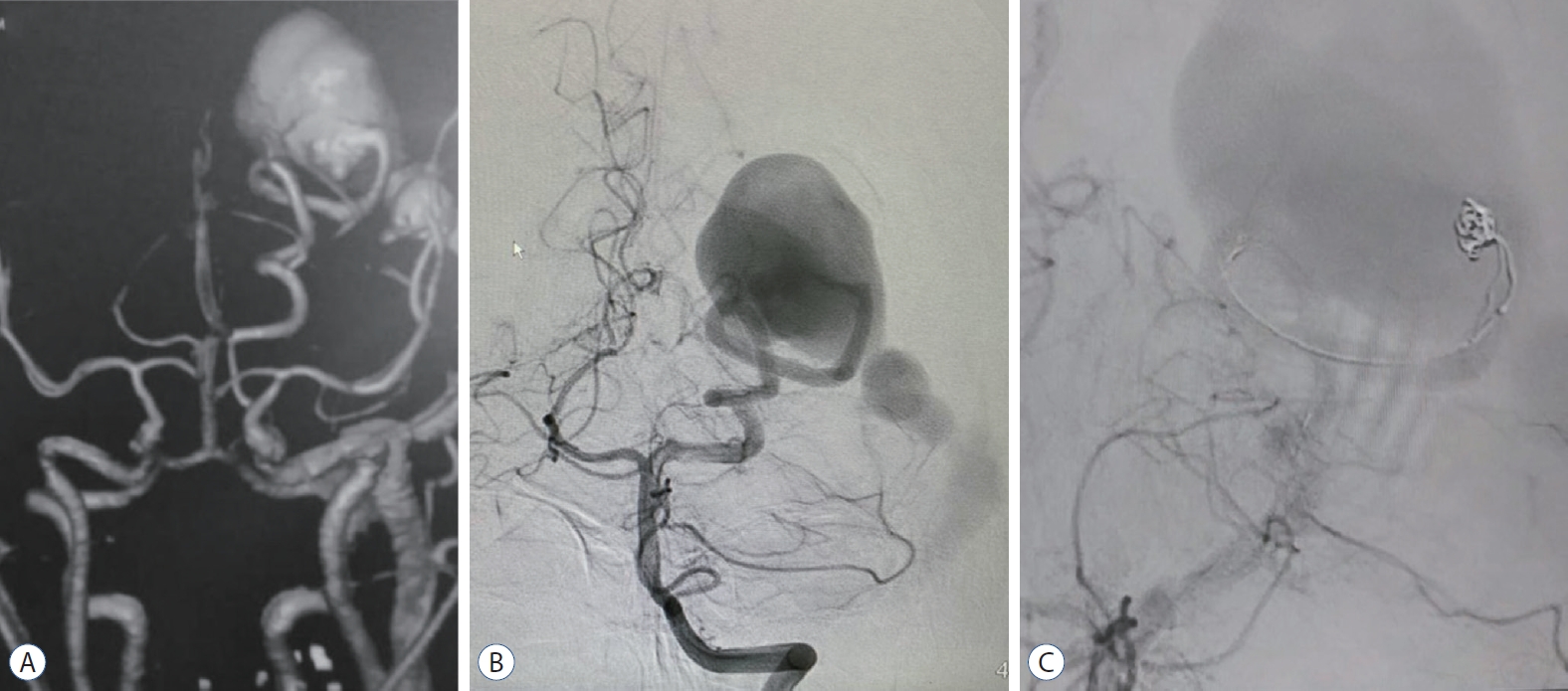
Case 2. A : Computed tomography angiography showing PAVF, supplied by hypertrophied posterior cerebral artery and aneurysmal dilatation of occipital vein with ectatic dural sinuses. B : Left vertebral artery angiogram (anteroposterior view) demonstrating PAVF, supplied by hypertrophied posterior cerebral artery and aneurysmal dilatation of occipital vein with ectatic dural sinuses. C : Left vertebral artery control angiogram (anteroposterior view) demonstrating attempted coil mass placement in the fistulous connection. PAVF : pial arterioveous fistula.
Two weeks later, the patient underwent TAE with onyx and arterial inflow control using a single lumen HyperGlide balloon applied proximal to the injecting Marathon flow-directed microcatheter. Onyx embolization of the fistulous junction and distal segment of the arterial feeder was accomplished. Control angiogram revealed complete occlusion of the shunt. Anticoagulants subcutaneous administration was continued for 10 days to avoid rapid and progressive thrombosis. Postprocedural, the patient, showed full resolution of the clinical manifestations, and follow-up radiology after 5 months revealed a significant reduction of the venous aneurysm and complete obliteration of PAVF (Fig. 4).
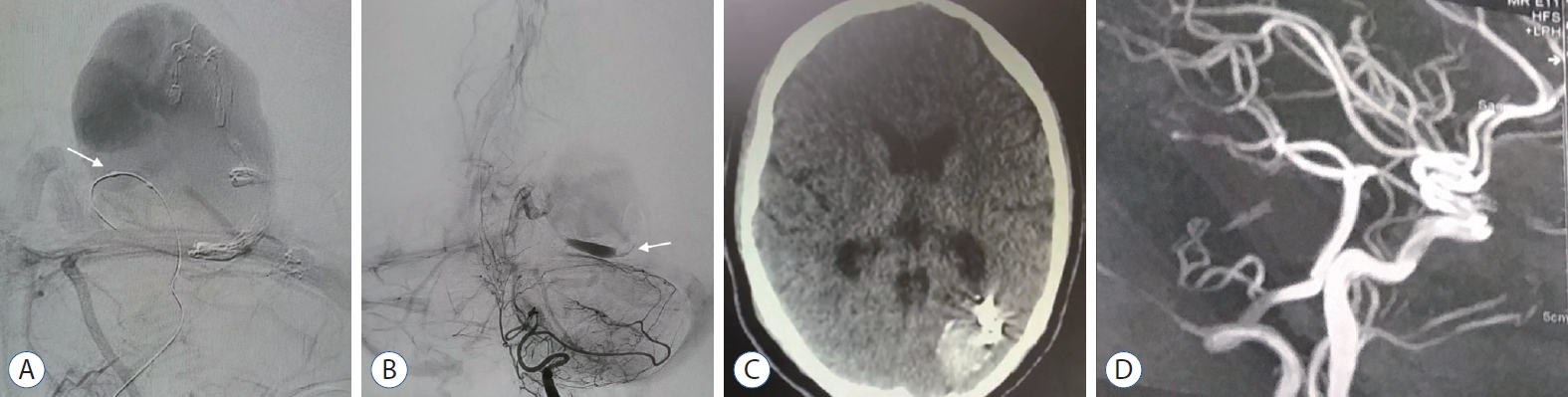
Case 2. A : Left vertebral artery control angiogram (anteroposterior view) demonstrating onyx embolized arterial feeder and fistulous connection, and deflated HyperGlide balloon proximally placed (white arrow). B : Final angiogram showing complete occlusion of the PAVF with onyx (white arrow). C : Follow up CAT scan of the brain showing onyx and reduced size venous aneurysm with partial thrombosis. D : Three-dimensional reconstructed angiographic done after 6 months, reveal no more filling of the PAVF. PAVF : pial arterioveous fistula.
Case 7
Female aged 7 years old manifesting epileptic fits for 3 months period. A DSA revealed a left parietal high flow PAVF between left middle cerebral artery (post central branch) and a cortical draining vein. The draining vein shows aneurysmal dilation measuring 4.5 cm in its widest dimension, with subsequent ectasia of the draining sinuses. The patient underwent TAE with glue to the fistulous connection and feeding artery; a Magic flow-directed microcatheter was navigated over Mirage 0.08 microguidewire to the fistulous connection, and subsequent NBCA glue mixture was injected slowly. The final angiogram showed complete obliteration of the PAVF (Fig. 5). Anticoagulants subcutaneous administration was continued for 10 days to avoid rapid and progressive thrombosis. Follow-up after 6 months revealed stable obliteration of the fistula and a significant decrease in venous aneurysm size.

Case 7. A : Left internal carotid angiogram (anteroposterior view) demonstrating PAVF, supplied by hypertrophied middle cerebral artery and aneurysmal dilatation of cortical draining vein in to superior sagittal sinus. B : Magnetic resonance imaging showing left high parietal PAVF and venous aneurysm. C : Selective angiogram with microcatheter, prior to embolization with n-butyl cyanoacrylate glue mix. D : Final angiogram showing the glue cast (white arrow). PAVF : pial arterioveous fistula.
Case 11
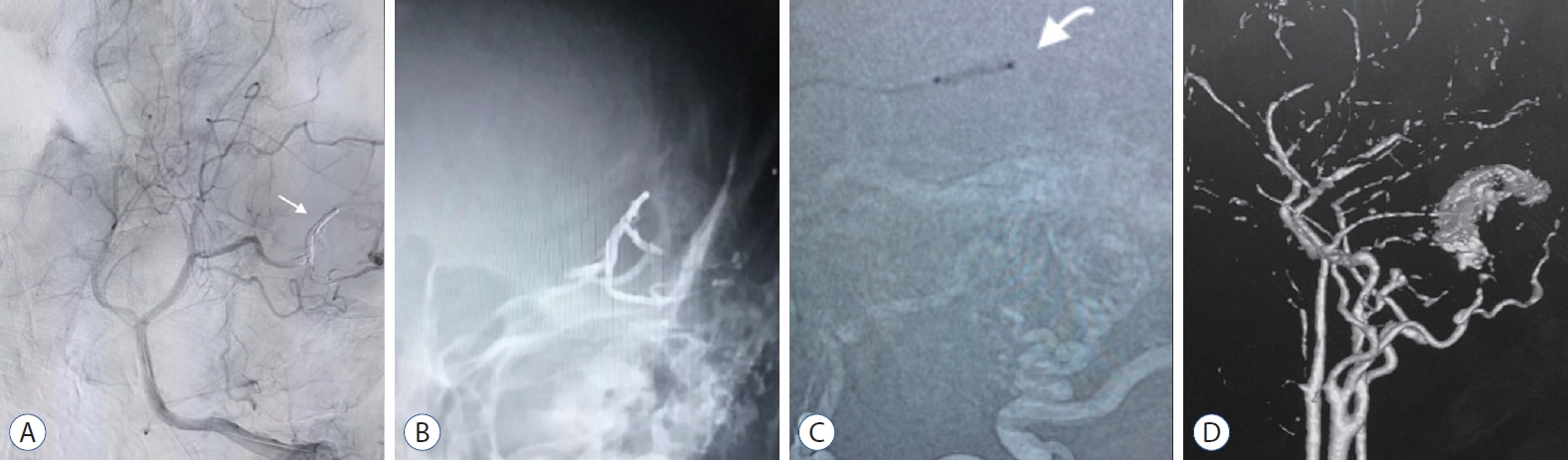
Male aged 55 years old was complaining of headache and tinnitus over 6 months period. The patient presented at the hospital with a disturbed conscious level, and a primary CAT scan brain revealed ICH. Surgical evacuation of the hematoma was done. Later on, DSA showed left transverse sinus Congard type III DAVF supplied by left MMA, internal maxillary artery, and OA meningeal branches. It drained into the transverse sinus, which was ectatic and associated with cortical venous ref lux. The patient underwent TAE with onyx and arterial inflow control using a Scepter C balloon catheter, allowing better penetration of onyx. The ICA control angiogram revealed disappeared arteriovenous shunting (Fig. 6). The patient restored his consciousness postprocedural, and follow-up confirmed stable obliteration of the fistula.
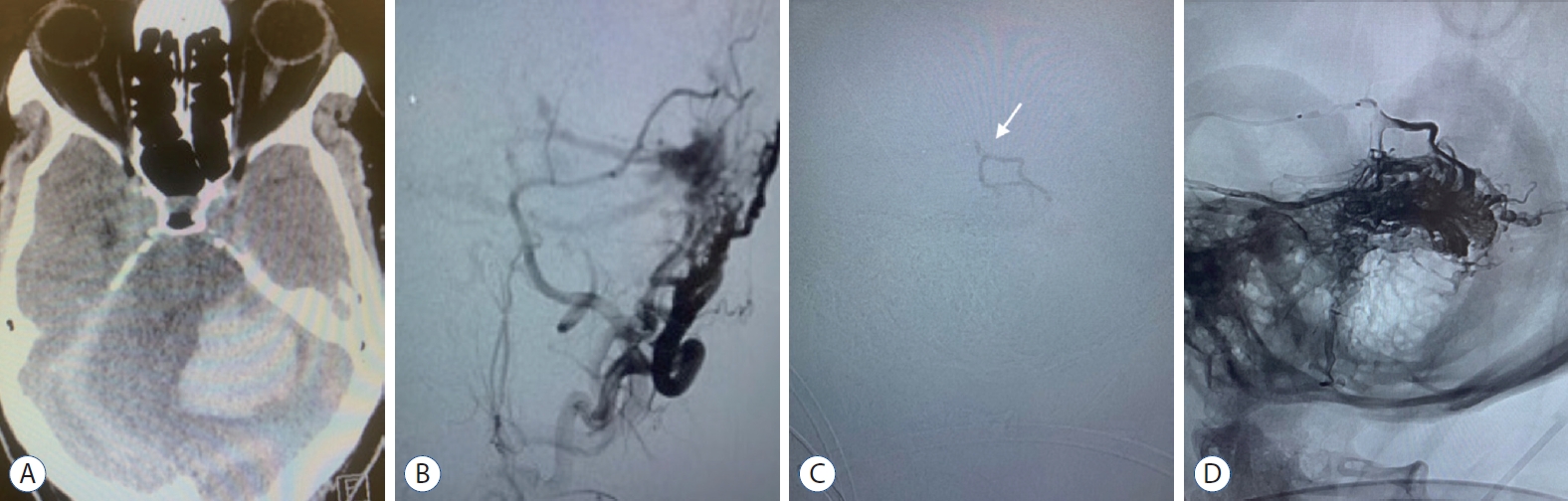
Case 11. A : CAT scan of the brain showing intracranial hemorrhage secondary to DAVF. B : Left external carotid angiogram (lateral view) demonstrating Cognard type IV DAVF, supplied by meningeal branches of middle meningeal, posterior auricular and occipital arteries draining into transverse sinus. C : Roadmap image showing onyx injection from the microcatheter (white arrow). D : Fluoroscopic unsubtracted roadmap image showing the onyx injection in to the DAVF. DAVF : dural arteriovenous fistula.
DISCUSSION
Treatment of high flow and aggressive intracranial AVFs
High flow and aggressive intracranial AVFs are usually associated with venous sinus thrombosis or venous hypertension, which in turn might be complicated with ICH [25]. In our study, ICH development was evident in six patients (40%), and that is why early management of these lesions is a must. Surgical treatment is one of the efficient treatment options, in recently published reports; the success rate of surgery for PAVFs and DAVFs was 96.8% and 83.3%, respectively, with no retreatment needed [23,27]. However, serious surgical morbidities cannot be neglected. In deeply situated fistulas with large venous varix, surgery could be a hazard. SRS has emerged as a potential treatment option. Though its role has not been conclusively clarified, as it requires a long duration for AVFs obliteration, and it is not preferred for fistulas with cortical venous drainage [9,22].
Endovascular embolization for intracranial AVFs
Endovascular embolization has become a gold standard treatment for intracranial AVFs over the years. Proper understanding of the complex angioarchitecture and variable hemodynamic is fundamental in selecting the most appropriate endovascular route for the obliteration of such type of intracranial fistulas. The transvenous approach is considered in fistulas with numerous and small arterial feeders [2]. However, in such category of AVFs that we included in this study, which are distantly located with fragile dilated or thrombosed venous outlet (Table 2), the endovenous navigation can be challenging, and the risk of perforation is properly high. TAE was efficient as a complete obliteration of AVFs using sole transarterial approach was 80% with the overall good functional outcome (mRS 0–2) 93.3%.
Evolution of TAE as a sole treatment and our experience
TAE is generally safe in our patients; technical complication (Alopecia errata), mostly related to overdose radiation exposure in two consecutive embolization sessions, was documented in only one patient (case 2). It has been reported that rapid progressive thrombosis occurs after the obliteration of AVFs with large venous pouches that can develop post-procedural ICH [10]. To guard against this devastating problem, we continued the anticoagulants administration regimen for 7–10 days in patients with high f low PAVFs and DAVFs with thrombosed dural sinus, and early follow-up CAT brain was mandatory.
Innovations in embolizing devices and techniques have helped in TAE technical feasibility [1,20]. That were evident in our included cases. TAE was accomplished in one session in 10 patients (66.7%). We used variable liquid embolizing agents (onyx, NBCA glue mixture, and squid) that were efficient in almost all of our cases. We used detachable and flow-directed microcatheters for proper navigation to the fistulous connection and safe delivery of embolizing agents. We applied the arterial inflow control technique in five (33.3%) of our included patients to guard against migration of such materials to the venous side, which could create an embolus or excessive thrombosis, and to accomplish better penetration of the embolizing material to the fistulous connection. We used singlelumen balloons and double-lumen balloons for that purpose (Table 2). Technical failure was documented in only one of our patients. We tried to apply an interlocking double microcatheter technique in (case 2) to achieve obliteration of the fistulous connection. Unfortunately, that was not successful as we described before. An important factor we did recognize from that two centers’ experience is that technical expertise played an important role in choosing the embolizing transarterial devices and techniques.
The residual was detected on DSA in only four patients (25%) after one session of TAE. One of them underwent another session of TAE. In the other three cases, we did consider using the transvenous approach later on. In compliance with no more arterial feeders suitable to seek, there was a significant reduction of the arteriovenous shunting and the venous outlet size in those cases, allowing more relative feasibility and safer circumstances to use the transvenous approach.
Limitations
Our study has some limitations; first, it is retrospective which might be prone to selection bias, and second, the variable angioarchitecture of the lesions, the different techniques applied, and the embolizing materials used might limit the analysis.
CONCLUSION
Endovascular embolization via transarterial approach for high flow or aggressive intracranial AVFs is a safe and considerable treatment option, especially for those associated with venous outlet ectasia and large venous varix or aneurysm that are challenging and relatively high-risk for TVE. In case of incomplete endovascular occlusion with TAE, the downgrading of these fistulas can be achieved, creating better circumstances for other complementary therapeutic options.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
INFORMED CONSENT
This type of study does not require informed consent.
AUTHOR CONTRIBUTIONS
Conceptualization : MAD
Data curation : MAD, SA, AEE
Formal analysis : MAD, SA, AEE
Funding acquisition : MAD
Methodology : MAD
Project administration : MAD
Visualization : MAD, SA, AEE
Writing - original draft : MAD, SA, AEE
Writing - review & editing : MAD, SA, AEE