Effect of Bevacizumab Treatment in Cerebral Radiation Necrosis : Investigation of Response Predictors in a Single-Center Experience
Article information
Abstract
Objective
Bevacizumab is a feasible option for treating cerebral radiation necrosis (RN). We investigated the clinical outcome of RN after treatment with bevacizumab and factors related to the initial response and the sustained effect.
Methods
Clinical data of 45 patients treated for symptomatic RN between September 2019 and February 2021 were retrospectively collected. Bevacizumab (7.5 mg/kg) was administered at 3-week intervals with a maximum four-cycle schedule. Changes in the lesions magnetic resonance image (MRI) scans were examined for the response evaluation. The subgroup analysis was performed based on the initial response and the long-term maintenance of the effect.
Results
Of the 45 patients, 36 patients (80.0%) showed an initial response, and eight patients (17.8%) showed delayed worsening of the corresponding lesion. The non-responders showed a significantly higher incidence of diffusion restriction on MRI than the responders (100.0% vs. 25.0%, p<0.001). The delayed worsening group showed a significantly higher proportion of glioma pathology than the maintenance group (87.5% vs. 28.6%, p=0.005). Cumulative survival rates with sustained effect were significantly higher in the groups with non-glioma pathology (p=0.019) and the absence of diffusion restriction (p<0.001). Pathology of glioma and diffusion restriction in MRI were the independent risk factors for non-response or delayed worsening after initial response.
Conclusion
The initial response of RN to bevacizumab was favorable, with improvement in four-fifths of the patients. However, a certain proportion of patients showed non-responsiveness or delayed exacerbations. Bevacizumab may be more effective in treating RN in patients with non-glioma pathology and without diffusion restriction in the MRI.
INTRODUCTION
Cerebral radiation necrosis (RN), typically manifesting as a necrotic white matter lesion, is one of the most dreaded toxicities associated with radiation therapies. The development of RN occurs secondary to radiation-induced endothelial cell damage-inducing upregulation of hypoxia-inducible factor 1-alpha with the subsequent release of vascular endothelial growth factor (VEGF) [25,33]. The release of VEGF results in increased vascular permeability, angiogenesis, and subsequently brain edema and inflammation, worsening neurologic signs and symptoms [3,8,10].
The most effective treatment options for RN include corticosteroids to relieve cerebral edema and surgical decompression to relieve mass effect, if any [34]. Corticosteroids counteract vascular endothelial damage and act by modulating inflammatory changes and edema, often leading to rapid symptomatic improvement after initiation [19,39]. Unfortunately, some patients do not benefit from corticosteroid therapy [21]. Alternative methods such as therapeutic anticoagulation, hyperbaric oxygen therapy, antiplatelet antibodies, laser interstitial thermal therapy, and high-dose vitamin E treatment have also been reported. Their efficacy, however, has yet to be proven [5,7,11,20].
Previous studies have revealed that overexpression of VEGF in resected RN lesions and the degree of radiation injury are correlated with the amount of VEGF expression [16,32]. Since preventing VEGF from reaching its capillary targets is a logical treatment strategy for RN, bevacizumab, a humanized monoclonal antibody against VEGF, might be an effective treatment option [12,31]. Gonzalez et al. [12] originally reported the efficacy of bevacizumab for treating RN in brain tumors. A placebo-controlled and double-blind, randomized trial performed by Levin et al. [24] showed that bevacizumab therapy markedly improved symptoms and signs in patients with RN. Despite the widespread optimism around the use of bevacizumab in the treatment of RN, the response and effect of bevacizumab in RN vary from case to case, with variable results. It would be useful for patients with RN and clinicians to understand the individual differences in bevacizumab response to provide the treatment benefit. We investigated the overall outcome of RN and factors that may lead to differences in initial response or maintenance after bevacizumab treatment.
MATERIALS AND METHODS
All data were anonymized, and the study was approved by the Institutional Review Board of Samsung Medical Center and performed in compliance with the ethical guidelines (approval No. 2022-01-136).
Patients
We conducted a retrospective search of electronic medical records in our institutional database. We identified 45 patients diagnosed as RN with lesion-related neurological symptoms and subsequently treated with bevacizumab at our institution between September 2019 and February 2021. All the patients had previously undergone various radiation therapies, including whole-brain radiation therapy (WBRT), fractionated local field radiation therapy, proton beam therapy, stereotactic radiosurgery (SRS), or a combination of these modalities. They received bevacizumab therapy after confirming that steroid treatment had not significantly improved symptoms or magnetic resonance image (MRI) findings. All treatment was performed after agreement in multidisciplinary consultation by a neurosurgeon, oncologist, and radiation oncologist. Demographic, clinical, and imaging data were obtained and retrospectively analyzed.
Diagnosis for RN
Currently, there are no definite clinical criteria to discriminate RN with certainty. Therefore, in our study, the diagnosis was based mainly on the radiographic findings from MRI scans. The comprehensive clinical features such as the patient’s treatment history and symptoms associated with the lesion were complementary findings.
The MR imaging for the diagnosis of RN was established through pre-gadolinium and post-gadolinium sequences and perfusion scans showing relative cerebral blood flow (rCBV). The morphological findings supporting the diagnosis of RN were based on the following characteristics : newly appeared or aggravated enhancing lesion without nodules within the radiation field in T1-weighted contrast-enhanced (T1-CE) image that can be characterized as “swiss cheese” or “soap bubble” appearance; a high signal in the T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) image in the brain parenchyma surrounding the lesion; no definite hyperperfusion in the perfusion scan (rCBV) [4,44]. For only selected equivocal cases that could not be diagnosed with MRI alone, a positron emission tomography scan with 18F-fluorodeoxyglucose and 11C-methionine tracer, or biopsy was performed.
Bevacizumab administration
Bevacizumab was administered to patients with a time interval of at least 3 months from prior radiation therapy, with no predisposing condition of bleeding, and who do not have evidence of lesion requiring urgent surgical intervention. Bevacizumab was given at a dose of 7.5 mg/kg. The regimen included up to a maximum four-cycle schedule in the absence of severe toxicity, with one infusion every 3 weeks.
Imaging protocol
Before starting bevacizumab treatment, all patients underwent MRI scans to establish a baseline imaging reference. The first follow-up images were collected after 4 weeks (i.e., after two cycles) from the start of bevacizumab treatment. Additional imaging was performed 3 weeks after the last bevacizumab treatment cycle (Fig. 1). Re-examinations were conducted every 2 to 3 months within 1 year, and then as per the patients’ condition, at intervals ≤6 months. For patients with intracranial symptoms, immediate re-examination was conducted. Data were reported up to September 2021.
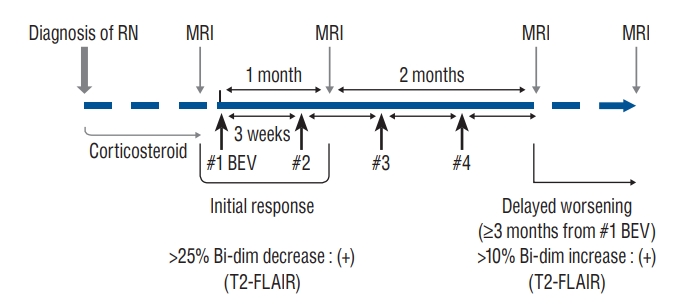
Schematic diagram of the bevacizumab treatment protocol for cerebral radiation necrosis. Bevacizumab at a 7.5 mg/kg dose was used to treat radiation necrosis. The regimen included up to a maximum four-cycle schedule in the absence of severe toxicity, with one infusion every 3 weeks. The response evaluation was performed after two cycles of bevacizumab therapy (4 weeks after the first bevacizumab administration). MRIs after 3 months from the first cycle of bevacizumab therapy were used to evaluate the durability of the effect of bevacizumab. RN : radiation necrosis, MRI : magnetic resonance image, BEV : bevacizumab, Bi-dim : bi-dimensional measurement, T2-FLAIR : T2-weighted fluid-attenuated inversion recovery.
Outcome evaluation
Responses were defined based on changes in radiographic findings and clinical symptoms. Radiographic changes were evaluated as bi-dimensional measurement, defined as the product of the longest diameter and its longest perpendicular diameter. Measures were then calculated and presented as a percentage change from the baseline images. Clinical data were also evaluated, including changes in dexamethasone dose and Eastern Cooperative Oncology Group performance status (ECOG PS) score.
The response evaluation performed after two cycles of bevacizumab therapy (4 weeks after the first bevacizumab administration) was defined as an “initial response”. We described an initial positive response as 1) a reduction in the bi-directional measurements on T2-FLAIR images by 25% and 2) no deteriorating symptoms. An initial negative response was defined as 1) a reduction in the bi-directional measurements on T2-FLAIR images by less than 25% or 2) any deteriorating symptoms. Follow-up imaging results were also reviewed to determine the delayed response of RN to treatment. MRIs after three months from the first cycle of therapy were used to evaluate the durability of the effect of bevacizumab. We defined “delayed worsening” as either 1) more than a 10% increase in the volume of the lesions on T2- FLAIR images over that of the last MRI, 2) the appearance of any new lesion/site, or 3) apparent neurological deterioration (Fig. 1).
Statistical analysis
IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA) and GraphPad Prism, version 8 (Graph Pad Software Inc., San Diego, CA, USA) were used for all statistical analyses. For categorical variables, data are expressed as frequencies and percentages. The chi-square test or Fisher’s exact test analyzed categorical variables using contingency tables. For continuous variables, data are expressed as the mean ±standard deviation. Continuous variables with normal distribution were analyzed using Student’s t-test. A logistic regression model was used to analyze the factors associated with risk for non-response or delayed worsening. Progression-free survival (PFS) was measured from the first bevacizumab to documented lesion worsening or the last follow-up. PFS was calculated using the Kaplan-Meier method. Differences between the survival curves were evaluated using the log-rank (Mantel-Cox) test. p-values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics of patients
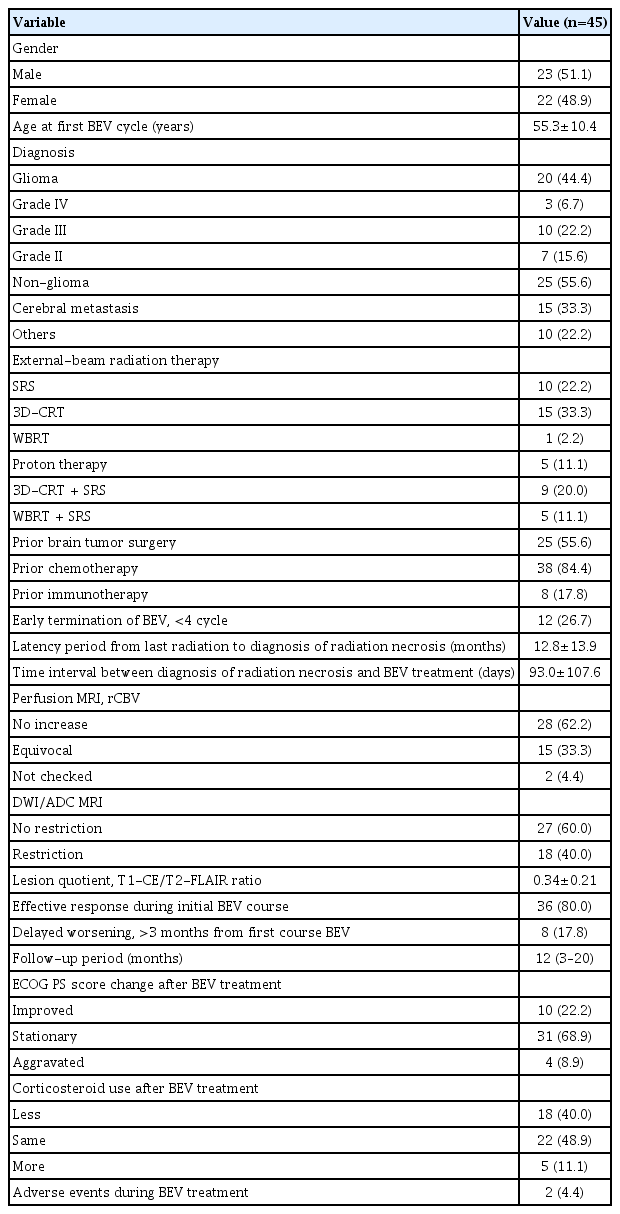
The study included 45 patients (22 women, 48.9%) with a mean age of 55.3±10.4 years. Twenty patients (44.4%) were diagnosed with glioma, and 25 patients (55.6%) were diagnosed with non-glioma pathology, including 15 patients (33.3%) with cerebral metastasis. Regarding radiation therapy modality, 10 (22.2%) received SRS, 15 (33.3%) received 3-dimensional conformal radiation therapy (3D-CRT), one (2.2%) received WBRT, and five (11.1%) received proton therapy. Nine patients (20.0%) received 3D-CRT and SRS, and five (11.1%) received WBRT and SRS. Of the included subjects, 25 (55.6%) had previously undergone brain tumor surgery, 38 (84.4%) had previously received chemotherapy, and eight (17.8%) had received immunotherapy (Table 1).
Of the 45 subjects, 12 patients (26.7%) ended bevacizumab treatment prematurely before reaching the schedule of four cycles. The latency period from the last radiation to the diagnosis of RN was 12.8±13.9 months and the time interval between diagnosis of RN and bevacizumab treatment was 93.0±107.6 days. There was no rCBV increase in 28 patients (62.2%) as observed using perfusion MRI, but equivocal (without hypermetabolism but ambiguous) rCBV findings were observed in 15 patients (33.3%). On diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) MRI, diffusion restriction was not observed within the enhancing boundaries in 27 patients (60.0%) but was observed in 18 patients (40.0%). The lesion quotient expressed by the T1-CE/T2-FLAIR ratio was a mean of 0.34±0.21 (Table 1).
Among all subjects treated with bevacizumab, 36 (80.0%) showed an effective response during the initial course. However, eight (17.8%) showed delayed worsening in follow-up MRI 3 months after the first cycle of bevacizumab therapy. The median follow-up period was 12 months (3 to 20 months) from the last bevacizumab cycle. Regarding the ECOG PS score, 10 patients (22.2%) showed improvement after bevacizumab treatment, 31 patients (68.9%) showed a fixed pattern, but four patients (8.9%) showed a worsening condition. The use of corticosteroids decreased in 18 patients (40.0%), remained unchanged in 22 patients (48.9%), and increased in five patients (11.1%) after bevacizumab administration. Adverse events (small non-fatal cerebral hemorrhages) occurred in two (4.4%) of 45 patients. Bevacizumab treatment was discontinued immediately after the side effects were confirmed (Table 1).
Comparison of the response-based subgroups
There were no significant differences between the two groups (responder and non-responder groups) in terms of gender, age, pathological diagnosis, and past tumor treatment history. Early termination was observed in eight cases (22.2%) from the responder group and four (44.4%) from the non-responder group, but no statistically significant difference was observed. Discontinued cases of the responder group included a case of small cerebral hemorrhage and seven cases of patient requests due to financial reasons. The latency period from the last radiation to the diagnosis of RN and time interval between diagnosis of RN and bevacizumab treatment were not significantly different between the two groups. Perfusion MRI scans showed equivocal rCBV findings in 10 patients (27.8%) from the responder group and five patients (55.6%) from the non-responder group. However, there was no statistically significant difference. In the case of diffusion restriction on MRI, there were nine patients (25.0%) from the responder group and nine patients (100.0%) from the non-responder group, showing a significant difference between the two groups (p<0.001). The lesion quotient was measured as 0.31±0.18 for the responder group and 0.45±0.29 for the non-responder group (Table 2).

Comparison of the responder group and the non-responder group of bevacizumab therapy after radiation necrosis
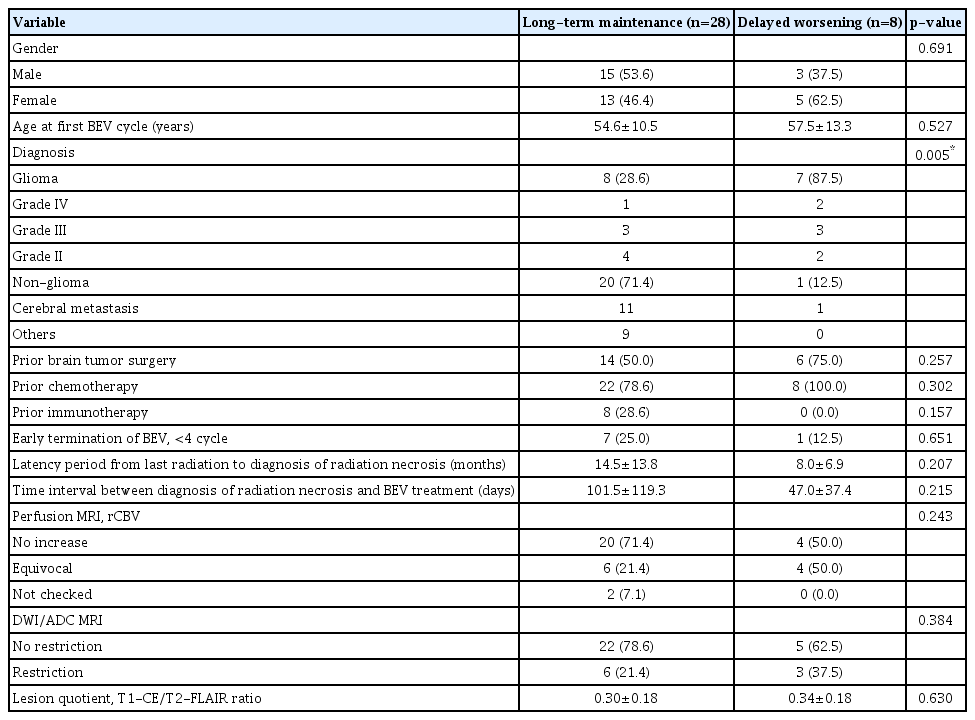
When comparing the long-term maintenance and delayed worsening groups, no significant differences were observed between the two groups in terms of gender and age. Comparing diagnosis proportions between the two groups showed that eight patients (28.6%) in the long-term maintenance group were diagnosed with glioma. In contrast, seven patients (87.5%) in the delayed worsening group showed a diagnosis of glioma (p=0.005). There were no significant differences between the two groups in the case of past tumor treatment history and early termination of bevacizumab treatment. There were no significant differences between the two groups in the latency period from last radiation to the diagnosis of RN, the time interval between diagnosis of RN and bevacizumab treatment, and MRI findings (Table 3).
The outcome of bevacizumab treatment according to pathology and diffusion restriction in magnetic resonance imaging
There was no significant difference in lesion quotient when comparing the glioma and non-glioma groups. However, the non-glioma group showed more significant improvement than the glioma group when comparing the T2-FLAIR images with baseline images during bevacizumab administration (after two cycles) (p<0.05), and T1-CE and T2-FLAIR images with baseline images after bevacizumab administration (3 months after the first cycle) (p<0.05) (Table 4).
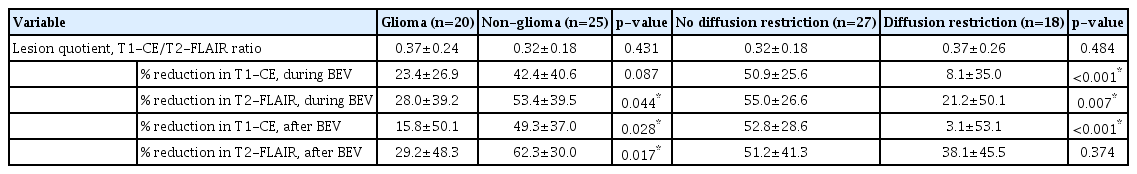
Radiographical outcome comparison of the subgroups according to pathology and diffusion magnetic resonance imaging
There was no significant difference in lesion quotient when comparing the group with and without diffusion restriction on MRI findings. However, no diffusion restriction group showed more significant improvement than the diffusion restriction group when comparing the T1-CE and T2-FLAIR images with baseline images during bevacizumab administration (p<0.05) and T1-CE images with baseline images after bevacizumab administration (p<0.001) (Table 4).
The impact of pathology and diffusion restriction in MRI on PFS after bevacizumab therapy for RN was evaluated with Kaplan-Meier curves. Kaplan-Meier estimates of PFS are presented in Figs. 2 and 3. Cumulative survival rates showing maintenance after bevacizumab treatment reached significance for the pathology of glioma (p=0.019) and diffusion restriction in MRI (p<0.001) via log-rank test.

Kaplan-Meier curve showing progression-free survival after bevacizumab therapy for radiation necrosis according to the pathology. Cumulative survival rates showing maintenance after bevacizumab treatment reached significance for the pathology of glioma (p=0.019) via log-rank test.

Kaplan-Meier curve showing progression-free survival after bevacizumab therapy for radiation necrosis according to restriction in the diffusion-weighted image. Cumulative survival rates showing maintenance after bevacizumab treatment reached significance for diffusion restriction (p<0.001) via log-rank test.
Risk factor analysis for response
Risk factors were evaluated concerning non-response or delayed worsening of the lesion. Pathology of glioma (odds ratio [OR], 6.000; 95% confidence interval [CI], 1.592–22.616; p=0.008), equivocal rCBV in perfusion MRI (OR, 3.750; 95% CI, 1.003–14.021; p=0.049), and diffusion restriction in DWI/ADC MRI (OR, 8.800; 95% CI, 2.215–34.965; p=0.002) showed significance in univariate binary logistic regression analysis. In multivariate analysis, pathology of glioma (OR, 6.054; 95% CI, 1.204–30.429; p=0.029) and diffusion restriction in MRI (OR, 14.197; 95% CI, 1.927–104.588; p=0.009) were the independent risk factors for non-response or delayed worsening after bevacizumab therapy for RN (Table 5).
DISCUSSION
Several studies have reported the therapeutic effect of bevacizumab in RN [4,29]. The reduction in brain edema volume varied from 49% to 63% in these studies. However, in clinical practice, variations in the response and effectiveness of bevacizumab are observed when treating RN on a case-by-case basis. According to the systematic review, about 15% of patients did not show a radiologic response even after bevacizumab administration, and about 34% of patients showed recurrence of the lesion [26]. We investigated the factors contributing to differences in response. Our results showed that restriction in the diffusion MRI and pathology of glioma was associated with non-responsiveness or delayed worsening of the lesion after bevacizumab therapy.
Diffusion imaging allows the evaluation of the rate of microscopic diffusion of free water molecules within tissues [1,40], and its magnitude is quantified with DWI and ADC [37]. Previously published studies have focused on the assessment of DWI/ADC values for assessing the diagnostic accuracy in differentiating tumor recurrence and RN [13,38,46]. It was expected that due to high cellularity, which restricts water mobility, tumor recurrence exhibits diffusion restriction and lower ADC value [6,13,38]. On the other hand, an increase in the ADC was expected due to water mobility from increased extracellular space associated with cell death in RN [6,13,38]. However, the sensitivity and specificity of DWI/ADC have not yet been fully characterized. The results have been inconsistent, with an ongoing debate on the use of diffusion restriction as a diagnostic tool in the RN [15,22,36,43]. A meta-analysis showing the diagnostic accuracy of diffusion MRI for differentiating RN and tumor recurrence showed a moderate diagnostic accuracy and opposed to using diffusion MRI alone to determine these two features [47]. Thus, we analyzed without including diffusion restriction as a diagnostic criterion.
Our results showed that diffusion restriction was related to non-responsiveness for bevacizumab therapy. We carefully hypothesized the possibility of the coexistence of delayed cytotoxic components from ongoing necrosis as a possible mechanism. In case of cell swelling due to ongoing necrosis, edema may occur through delayed cytotoxicity [17,18,28]. The ADC value decreases as the cells expand due to the narrowing extracellular space within the brain parenchyma [30]. From the mixed properties of the cytotoxic edema with vasogenic edema, the effect of bevacizumab may be weakened, which mainly acts on vasogenic edema [30].
Another possible hypothesis is that of residual active disease or tumor cell repopulation by resistant cells. Although pathological confirmation is the gold standard for diagnosing RN, such confirmation cannot be achieved easily in the clinic. Comprehensive imaging-based diagnosis is the most practical and most commonly applied method although primarily imaging-based determination cannot exclude the possibility that a small number of living tumor cells are present in or around the lesion. When the viable tumor is mixed with RN, an overlap of diffusion restriction can occur [2,13,14,35,41,45], particularly in cases with infiltrative pathology (e.g., glioma), and may have possibly resulted in a difference in the bevacizumab response in RN, which shows a diffusion-restriction pattern. The limitation that the pathology of the corresponding lesions were not analyzed in the present study, this should be taken into consideration when interpreting the results.
In our study, the pathology of glioma was associated with delayed worsening of the lesion. There have been a few previous reports of responses to bevacizumab treatment for RN in patients with glioma [9,27,42]. Dahl et al. [9] reported results of bevacizumab treatment in seven patients with glioma with RN in ages ranging between 1 and 25 years. The median follow-up was four months (ranging from 6 weeks to 21 months), with increased necrosis in four of seven patients [9]. Liu et al. [27] reported that out of four children with pontine glioma who received bevacizumab as a treatment for the RN, one child did not respond to bevacizumab and showed disease progression. Torcuator et al. [42] reported the results of bevacizumab treatment for six patients with glioma with biopsy-proven RN. After administration of bevacizumab, radiological responses were found in all patients [42]. However, three patients died due to tumor progression [42]. It was demonstrated that the patients with glioma pathology were associated with faster worsening of the lesion despite bevacizumab treatment. However, it was difficult to infer a valid reason for these phenomena in our study. It was presumed to be related to the pathobiological characteristics of the tumor, but this should be elucidated in further research.
Bevacizumab, despite its therapeutic efficacy, can induce several adverse events, of which central nervous system (CNS) hemorrhages constitute a potentially fatal complication. In this study, two (4.4%) of 45 patients had small cerebral hemorrhages. The frequency of hemorrhagic side effects in this study is relatively consistent with that in the recent literature, which indicates an overall incidence of CNS bleeds in 1.2–4.6% of patients who are receiving bevacizumab [23]. However, the retrospective nature of this study could have resulted in other non-overt complications being neglected. Although both cases in this study comprised non-fatal lesions, bevacizumab for RN should be administered while exercising caution in regard to the possibility of adverse events.
Our study has some limitations. The diagnosis of RN still has ambiguity. Although advanced imaging techniques provided valuable information, there was no histopathological verification of the final diagnosis. Thus, imaging evidencing RN could not exclude the possibility of viable tumor cells. When the RN lesion shows delayed exacerbation, especially in the case of gliomas, it was challenging to make a clear time point of lesion progression, with difficulty in clearly distinguishing the lesion worsening from the tumor progression recurrence. In addition, we acknowledge that the follow-up period of each case was heterogeneous due to the study design. Well-designed prospective studies comparing the available imaging and histopathology as a valid reference are needed in the future.
CONCLUSION
Bevacizumab showed fairly effective mitigation of edema in RN. However, differences occurred in responses. We propose that bevacizumab may be more effective in patients without diffusion restriction in MRI and those with non-glial tumors. The cause of the difference in the response of bevacizumab should be elucidated by further studies supporting a biological mechanism.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
This type of study does not require informed consent.
Author contributions
Conceptualization : JIL; Data curation : SHL, JWC, DSK, HJS, DHN, JIL; Formal analysis : SHL; Methodology : SHL, JIL; Project administration : JIL; Visualization : SHL; Writing - original draft : SHL, JIL; Writing - review & editing : SHL, JWC, DSK, HJS, DHN, JIL
Data sharing
None
Preprint
None