Choroid Plexus Hyperplasia : Report of Two Cases with Unique Radiologic Findings
Article information
Abstract
Choroid plexus hyperplasia (CPH), also known as diffuse villous hyperplasia of choroid plexus, is a rare condition characterized by excessive production of cerebrospinal fluid (CSF), resulting in hydrocephalus. Diagnosing CPH can be challenging due to the absence of clear imaging criteria for choroid plexus hypertrophy and the inability to assess CSF production non-invasively. As a result, many CPH patients are initially treated with a ventriculoperitoneal (VP) shunt, but subsequently require additional surgical intervention due to intractable ascites. In our study, we encountered two CPH patients who presented with significantly enlarged subarachnoid spaces, reduced parenchymal volume, and prominent choroid plexus. Initially, we treated these patients with a VP shunt, but eventually opted for endoscopic choroid plexus cauterization (CPC) to address the intractable ascites. Following the treatment with endoscopic CPC, we observed a gradual reduction in subarachnoid spaces and an increase in parenchymal volume. In cases where bilateral prominent choroid plexus, markedly enlarged subarachnoid spaces, and cortical atrophy are present, CPH should be suspected. In these cases, considering initial treatment with combined endoscopic CPC and shunt may help minimize the need for multiple surgical interventions.
INTRODUCTION
The etiologies of hydrocephalus are diverse and extensively studied in the literature. The cause of hydrocephalus can be crudely classified into abnormal production, abnormal circulation, and abnormal absorption of cerebrospinal fluid (CSF) or their combinations. Regarding the production of CSF, the choroid plexus is known to constitute a major proportion of the total amount [3]. Choroid plexus hyperplasia (CPH), also known as diffuse villous hyperplasia of choroid plexus, is a rare cause of CSF overproduction that leads to hydrocephalus [1,16]. The characteristics of CPH include diffuse hypertrophy of choroid plexus and CSF overproduction, but the diagnosis can be somewhat challenging. There are no clear imaging criteria of choroid plexus hypertrophy, and the amount of CSF production cannot be assessed with non-invasive measures [2]. The scarcity of literatures on the CPH entity contributes to limited knowledge of the disease. Herein we present two cases of CPH patients with characteristic feature of decreased parenchymal volume and enlarged subarachnoid spaces, along with prominent choroid plexus. Quantitative volumetric analysis was performed with magnetic resonance imaging (MRI) using dedicated image analysis software (Xelis 1.0.6; INFINITT Healthcare Co., Ltd., Seoul, Korea). Parenchymal volume was calculated by subtracting CSF volume from the intracranial volume. The percentage of parenchymal volume to intracranial volume is presented. We believe that our report will contribute to the understanding and management of CPH.
CASE REPORT
This study protocol was approved by the Local Institutional Review Board (IRB, No. 1703-066-839) and was conducted according to the Helsinki Declaration. The requirement for written informed consent was waived due to the retrospective design of this study, as determined by the IRB.
Patient 1
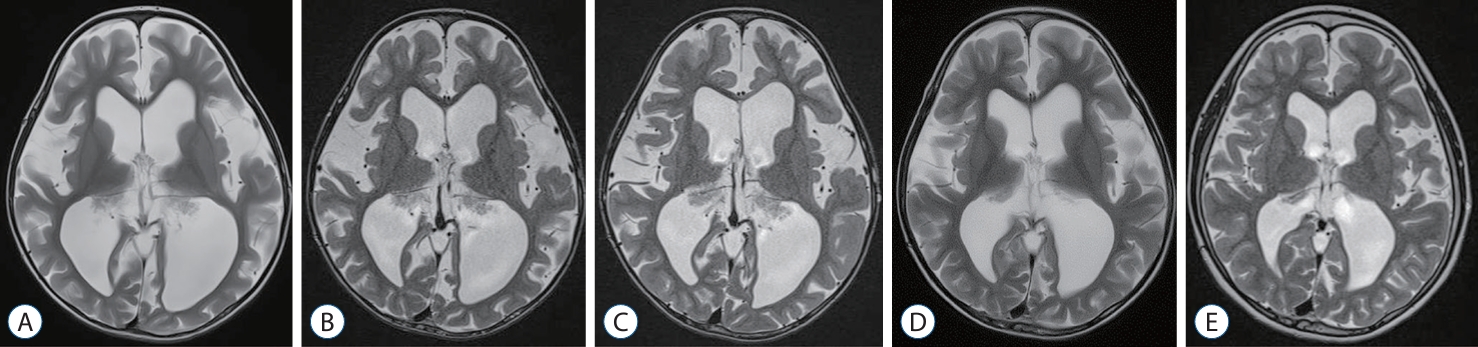
A 5-month-old girl was referred to our center due to a large head circumference (HC) (43.3 cm, 75–90th percentile for her age) (Fig. 1A). She was born full-term, weighed 2.85 kg and had an unremarkable medical history. Her HC at birth was 30 cm (<5th percentile). Follow-up with brain ultrasonography revealed no interval changes. At 14-months of age, her HC was 48.5 cm (>97th percentile), and the MRI showed slightly prominent choroid plexus with ventricle enlargement (Fig. 1B). At 31-months of age, her anterior fontanelle was soft and sunken down, but her HC increased to 55.45 cm (>97th percentile) and she showed gross motor developmental delay. The MRI revealed relatively prominent choroid plexus with marked progression of ventriculomegaly, enlargement of the subarachnoid space, and decreased parenchymal volume (Figs. 1C and 2A). Although she had no signs of acute increased intracranial pressure due to the open fontanelle, the decision was made to perform a ventriculoperitoneal (VP) shunt due to increased HC and ventriculomegaly. A VP shunt insertion with Strata adjustable pressure valve (Medtronic, Minneapolis, MN, USA) was performed, and the opening pressure was 36 cm CSF.

Radiologic findings of patient 1. A 5-month-old girl presented with a large head circumference. A : The initial magnetic resonance imaging (MRI) shows a slight enlarged subarachnoid space. B : At 14-month of age, a prominent choroid plexus is observed. C : At 31-month of age, ventriculoperitoneal (VP) shunt was performed due to significant progression of ventriculomegaly, enlargement of the subarachnoid space, and decreased parenchymal volume. D : After the VP shunt, the patient experienced intractable ascites, and at 44-month of age, progressive ventriculomegaly is evident, leading to endoscopic choroid plexus cauterization. E and F : The MRI scans at 63-month of age and 10-year follow-ups show recovery of the parenchymal volume and resolution of ventriculomegaly.
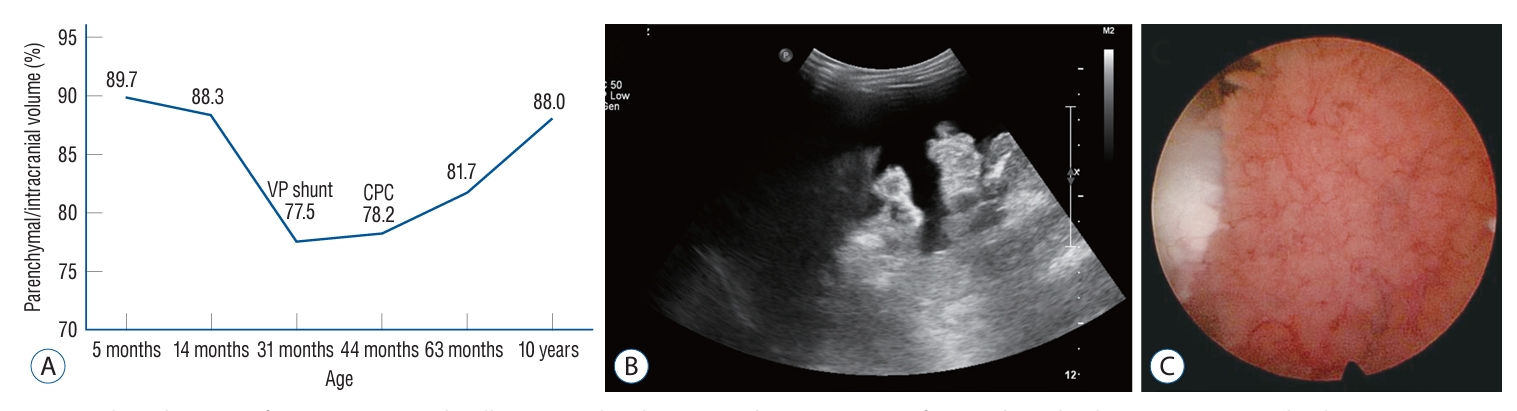
Clinical course of patient 1. A : A plot illustrating the changes in the percentage of parenchymal volume to intracranial volume over time in patient 1. B : Abdomen ultrasonography shows intractable ascites occurred following the ventriculo-peritoneal shunt. C : Intraoperative photo during the endoscopic choroid plexus cauterization displaying the hyperplastic choroid plexus during endoscopic choroid plexus cauterization. VP : ventriculo-periotneal, CPC : choroid plexus cauterization.
After the shunt, there were no signs of improvement, and a large amount of intractable ascites caused abdominal distension (Fig. 2B). VP shunt was left untouched. Eventually, the patient decided to undergo endoscopic choroid plexus cauterization (CPC) at 45 months of age (Fig. 1D). Endoscopic CPC was performed using a rigid endoscope and an endoscopic bipolar instrument. The patient was placed in the prone position. Two parieto-occipital burr holes were made on each side. The endoscope was introduced through the atrium of the lateral ventricle. Cauterization was performed along the choroid plexus. Subtotal cauterization of the choroid plexus was carried out because the temporal horn was not accessible due to the use of a rigid endoscope (Fig. 2C). The postoperative course was uneventful with resolution of the intractable ascites, and her gross motor developmental delay fully recovered at 63 months old (Fig. 1E). At the last follow-up at 10 years old, she was unremarkable except for mild cognitive function impairment, her VP shunt was functioning, and the MRI showed marked recovery of the parenchymal volume (Figs. 1F and 2A).
Patient 2
A 31-month-old girl was referred due to a large HC (55.4 cm, >97th percentile) and gross motor developmental delay. She was born full-term, weighed 3.5 kg and had an unremarkable medical history. Her HC at birth was unknown. Her initial MRI revealed a prominent choroid plexus with marked enlargement of the subarachnoid space, and cortical atrophy without obstructive lesions (Figs. 3A and 4A). Because of the clinical experience of the patient in patient 1, we recommended endoscopic CPC as the first treatment. However, the patient’s caregivers refused and preferred to have VP shunt surgery. A VP shunt insertion with Strata adjustable pressure valve (Medtronic) was performed with an initial setting of 1.0 performance level, and the opening pressure was 21 cm CSF.
Radiologic findings of patient 2. A 31-month-old girl presented with a large head circumference and gross motor developmental delay. A : Her initial magnetic resonance imaging (MRI) reveals a prominent choroid plexus with marked enlargement of the subarachnoid space and cortical atrophy. B : At 38-month of age, no changes in the enlarged subarachnoid space and decreased parenchymal volume are observed. C : At 44-month of age, enlarged subarachnoid space and decreased parenchymal volume is still present and the endoscopic choroid plexus cauterization was performed. D : At 51-month of age, the MRI shows a slight increase in parenchymal volume. E : At the latest follow-up at 8 years old, the patient fully recovered from the developmental delay, and the MRI reveals a marked increase in parenchymal volume.

Clinical course of patient 2. A : A plot illustrating the changes in the percentage of parenchymal volume to intracranial volume over time in patient 2. B : Abdomen ultrasonography demonstrates intractable ascites occurred following the ventriculo-peritoneal shunt. C : Intraoperative photo the endoscopic choroid plexus cauterization showing the hyperplastic choroid plexus with choroidal vessel. VP : ventriculo-periotneal, CPC : choroid plexus cauterization.
Abdominal distension occurred immediately after the shunt, so we changed the shunt setting to 2.0 performance level (Figs. 3B and 4B). Six months after the VP shunt, an inguinal hernia occurred, requiring surgical repair. The decision to perform endoscopic CPC was made since the abdominal distension persisted 6 months after the inguinal hernia operation at 44-months of age (Fig. 3C). Endoscopic CPC was performed in the same manner as described above (Fig. 4C). After endoscopic CPC at 51-months of age, the abdominal distension improved immediately, and the MRI showed a slightly increased parenchymal volume (Fig. 3D). At the last follow-up at 8 years old, she fully recovered from her developmental delay, her VP shunt was functioning, and MRI revealed an improvement in parenchymal volume (Figs. 3E and 4A).
DISCUSSION
CPH is a very rare condition, which has been reported in no more than 30 cases in the literature since its first description in 1924 [4,7]. However, due to its distinctive features and unique characteristic of causing hydrocephalus through overproduction of CSF, CPH is a renowned disease entity in the field of hydrocephalus research [12]. Among the several factors relevant to the pathogenesis of hydrocephalus, the choroid plexus plays a key role [15].
The treatment of CPH has been comprehensively discussed in previous studies. Diverse treatment options, such as ventriculoatrial shunts and choroidal artery embolization, have been suggested in a few reports [10,11]. CPC is accepted as the mainstay of CPH treatment in many studies since the pathophysiologic cause of hydrocephalus is predominantly CSF overproduction [8,13,14]. CPH patients who were initially treated with a VP shunt without CPC required subsequent surgical treatment due to intractable ascites [4]. This is because the recognition of CPH upon encountering the patient is difficult. However, it should be noted that in most cases in the literature where patients were also treated with a shunt, CPC alone may not be sufficient to normalize the increased intracranial pressure.
The diagnosis of CPH is challenging due to several characteristics of the disease. There are no radiologic criteria that define the hyperplasia of the choroid plexus [2]. Additionally, CSF overproduction, which is a hallmark of CPH, must be confirmed but the amount of CSF production can only be assessed by invasive measures. Another reason for the poor recognition of the disease is that the severity of the CPH seem to vary. In some patients, observation alone was sufficient, and no treatment was required at al [l5]. CPH must also be distinguished from tumorous conditions, such as choroid plexus papilloma and choroid plexus carcinoma, by the absence of nodular, lobulating mass [6].
From the review of our two CPH patients, we have noted that both cases commonly presented markedly enlarged subarachnoid spaces in combinations with cortical atrophy. The overproduction of CSF can result in an enlarged subarachnoid space and ventricle, resulting in parenchymal volume loss. Additionally, after treatment of CPH, a gradual decrease in subarachnoid spaces with an increase in parenchymal volume was also notable. The imaging feature of enlarged subarachnoid space has been suggested in another paper [6]; however, its relation to parenchymal volume is first described in this study.
In fact, an enlarged subarachnoid space is a relatively common finding in infants. Benign enlarged subarachnoid space (BESS), also known as benign external hydrocephalus is a well-recognized benign condition [9]. In patient 1, it was not distinguishable from BESS at the first clinic visit at 5 months. However, since BESS has a self-limiting course and usually resolves around 12 months of age, BESS can be excluded in cases with a late presenting age and an aggravating clinical course on follow up [17].
The absence of measured CSF production amount is one of the limitations in our study. In addition, no tissue confirmation and no information on proliferative index of choroid plexus lead to limited information. However, the absence of a mass-like lesion in the choroid plexus and confirmation of CSF overproduction by intractable ascites were sufficient for a confirmed diagnosis of CPH.
CONCLUSION
We conclude that the presence of bilateral prominent choroid plexus, along with markedly enlarged subarachnoid spaces and decreased parenchymal volume, suggests CPH. In these cases, initial treatment with combined endoscopic CPC and shunt should be considered to avoid multiple surgical interventions.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
This type of study does not require informed consent.
Author contributions
Conceptualization : JHP; Data curation : JWK, WH; Formal analysis : JWK, WH; Funding acquisition : JHP; Methodology : JHP, SKK; Project administration : JHP, SKK; Visualization : JWK, WH; Writing - original draft : JWK, WH; Writing - review & editing : JHP, SKK
Data sharing
None
Preprint
None
Acknowledgements
This study was supported by a grant from SNUH Kun-hee Lee Child Cancer & Rare Disease Project, Republic of Korea (No. 22A-003-0100; to Phi JH).
