Novalis Stereotactic Radiosurgery for Spinal Dural Arteriovenous Fistula
Article information
Abstract
The spinal dural arteriovenous fistula (SDAVF) is rare, presenting with progressive, insidious symptoms, and inducing spinal cord ischemia and myelopathy, resulting in severe neurological deficits. If physicians have accurate and enough information about vascular anatomy and hemodynamics, they achieve the good results though the surgery or endovascular embolization. However, when selective spinal angiography is unsuccessful due to neurological deficits, surgery and endovascular embolization might be failed because of inadequate information. We describe a patient with a history of vasospasm during spinal angiography, who was successfully treated by spinal stereotactic radiosurgery using Novalis system.
INTRODUCTION
The spinal dural arteriovenous fistula (SDAVF) is the most common type of spinal vascular malformation, but it is rare condition in overall incidence561013171820). It is characterized by progressive, insidious, and non-specific symptoms that are similar to those of more common etiology, such as degenerative spinal disorder and peripheral neuropathy5131920). The fistulas of SDAVF are located intradurally at the sleeve of the nerve root, and the obliteration of fistulas is the treatment goal for SDAVF513182223). SDAVF can be treated by surgical interruption and endovascular embolization. To help ensure successful treatment, selective spinal angiography is necessary to provide information about vascular architectures561215181922). However, spinal angiography has been shown to cause paraparesis in several cases68151617).
We present a patient with SDAVF and a history of paraparesis during spinal angiography who was treated successfully using stereotactic radiosurgery (SRS) using Novalis system.
CASE REPORT
A 43-year-old man presented with slow progression of back pain, voiding difficulty, and boring pain on both lower extremities during 5 months. His symptoms worsened 5 days prior to his presentation to our hospital. Neurological examination showed 420motor weakness of grade IV in both legs and his anal tone was grade zero. Sensory changes, such as hypesthesia and analgesia were presented below the T9 level. About 14 years ago, he had pain in the left leg and was diagnosed with spinal arteriovenous malformation. At that time, magnetic resonance imaging (MRI) of the thoracolumbar spine revealed a vascular anomaly at the thoracolumbar level, and physicians performed spinal angiography for definite treatment. However, spinal angiography had failed three times in two other hospitals due to vasospasm and paraparesis. He recovered from these symptoms spontaneously, and he had not undergone any treatment thereafter.
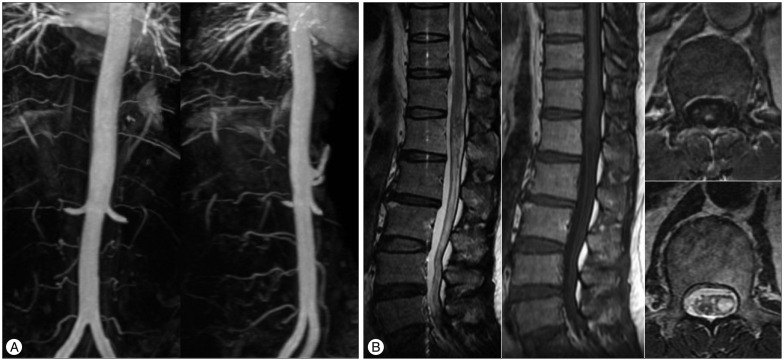
MRI in our hospital showed a vascular anomaly with enhancement at the T12, L1, and L2 levels (Fig. 1A). Axial images of the L1 level revealed a mass lesion, located on the left side of the intraspinal canal (Fig. 1B). We recommended selective spinal angiography, but the patient refused because of his experience, requesting a non-invasive technique for diagnosis and treatment. We planned SRS using the Novalis system (BrainLAB®, Heimstetten, Germany). However, we required an alternative modality to selective spinal angiography for obtaining accurate information on diagnosis, level of the lesion, and follow-up after treatment. Thus, a three-dimensional volumetric sagittal time-resolved imaging of contrast kinetics (TRICKS) abdominal magnetic resonance angiography (MRA) using 1.5T MRI system were performed in quiet respiration (TR/TE/flip=4.2/1.1/45°, FOV 330×330, equivalent slice thickness of 3 mm, matrix 256×160). Contrast medium (15 mL gadolinium) was administrated. The study was post-processed into maximum-intensity projection (MIP) images (Fig. 1C). These images showed the SDAVF feeding from the L1 lumbar artery. Magnetic resonance myelography showed a left-sided vascular anomaly (Fig. 1D). SRS through the Novalis system (BrainLAB®) was performed. The target, involving the dura margin, was constructed to include the fistula during structural segmentation under spinal computed tomography (CT) images (Fig. 2A). The volume of the lesion was 2.533 cc. We used 10 conformal beams and a total irradiation of 18 Gy with three fractions (Fig. 2B).
Magnetic resonance imaging (MRI) of the thoracolumbar spine reveals abnormal lesions with signal void and enhancement, indicating a vascular anomaly at the T12, L1, and L2 levels (A). Axial images at the L1 level show a left-sided mass lesion in the spinal canal (B). Three-dimensional volumetric contrast-enhanced sagittal TRICKS abdominal magnetic resonance angiography (MRA) using 1.5T MRI system was performed. The study was post-processed into maximum-intensity projection images. These images show a feeding artery at the L1 level (C). Magnetic resonance myelography (anterior-posterior view) reveals a left-sided vascular anomaly (D).
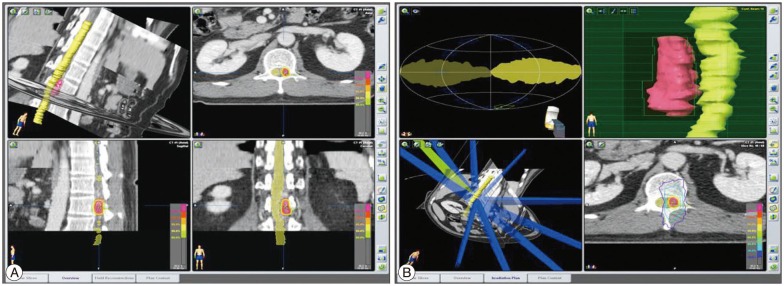
View of the treatment plan. The target, involving the dura margin, was constructed to include the fistula during structural segmentation under spinal computed tomography imaging (A). Ten conformal beams were used, and a multileaf collimator shaped the target margin. The yellow isodose line represents the 90% isodose line and total prescribed dose of 18 Gy. The blue line represents the 30% isodose line (6 Gy). This treatment plan showed a low dose of radiation exposure to spinal cord (B).
The patient's back pain improved 7 months after the treatment. Three years later, the patient could walk unassisted. But the voiding problem remained.
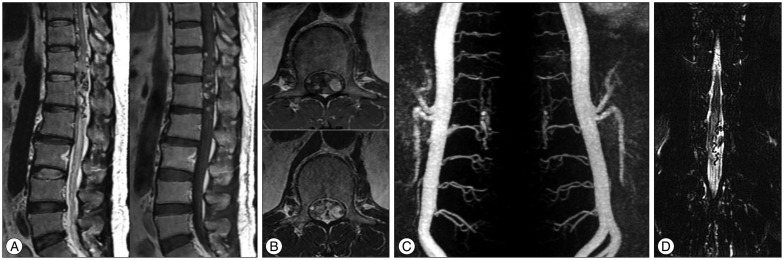
We checked the three-dimensional sagittal TRICKS abdominal MRA using 1.5T for follow-up 7 months after SRS, and three-dimensional sagittal TRICKS abdominal MRA using 3.0T MRI system (TR/TE/flip=10.0/1.5/30°, FOV 380×380, equivalent slice thickness of 4 mm, matrix 384×160) was performed 3 years after radiosurgery. The size of the spinal lesion was decreased and the flow through the fistula was diminished on the 7-month post-treatment images (Fig. 3). Three years after SRS, the feeding artery for the SDAVF disappeared (Fig. 4A), and the sequelae of the previous lesion was presented on follow-up MRI and MRA images (Fig. 4B).
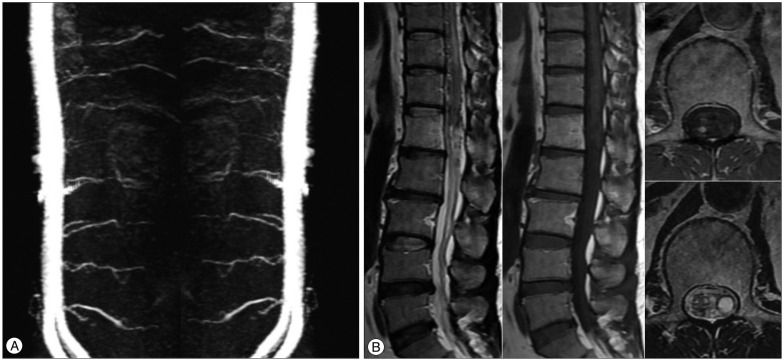
Follow-up MRI and MRA images after 7 months. The size of the feeding artery and abnormal vascular lesion is decreased (A). The previous lesion with signal void and enhancement is also reduced (B).
DISCUSSION
SDAVF represent a rare pathological condition, but they account for 60–80% of all spinal vascular malformation61317182023). They are acquired lesions, and usually present with insidious and progressive symptoms, such as paraparesis and sensory deficits of the bladder, bowel, and lower extremities10131718). As they present with non-specific and misleading clinical symptoms, the final diagnosis is sometimes delayed from the onset of neurological deficit561213171923). Their most common site is the thoracolumbar region, and the arteriovenous shunt is a low-flow shunt located at the dural sleeve of the spinal nerve root56101323). The pathophysiology of SDAVF-induced spinal cord ischemia and myelopathy is due to increased venous pressure, venous congestion, and decreased spinal cord perfusion caused by shunting arterial blood into the venous side56121315171823). Treatment methods for these lesions are microsurgery and endovascular embolization. Because the selection of treatment method is mediated by the physician's preference, the optimal treatment remains controversial523).
Selective spinal angiography is regarded as an essential procedure to confirm and treat SDAVF5). For successful surgery or endovascular coiling, selective spinal angiography is necessary to know the architectures and hemodynamics of the lesion561215181922). However, even with experienced neuro-interventionists, selective spinal angiography can sometimes cause complications from vasospasm, increased venous pressure, and spinal cord infarction6812151720). In our case, the patient experienced paraparesis three times during spinal angiography. Although it occurred 14 years previously, the patient refused the spinal angiography. Because the patient wanted a noninvasive method for diagnosis and treatment, we found an imaging technique to provide information about the lesion and be available for follow-up. There are some advanced imaging techniques for SDAVF, such as contrast enhanced magnetic resonance angiography with high resolution, TRICKS sequence, gradient echo, and spinal angiography with a 256-slice CT23152024). Although the role of advanced imaging techniques is limited to reducing the exposure of contrast and radiation during the subsequent selective spinal angiography61215202124), it is sufficient for diagnosing SDAVF and localizing the fistula235152024). Of these imaging techniques, we used the three-dimensional sagittal TRICKS abdominal MRA to obtain information about the spinal lesion and plan the SRS. We utilized TRICKS sequence for follow-up to confirm therapeutic effects.
SRS for SDAVF is not an established method, so reports regarding SDAVF treated by SRS are very rare. However, in terms of radiosurgery for cranial dural arteriovenous fistulas, radiosurgery for SDAVF is not an impossible treatment option22). Gao et al.7) reported that high expression state of endothelial progenitor cells (EPCs) presented in the brain and spinal arteriovenous malformation (AVM) tissue. Other authors have published the results from an experimental study that radiosurgery decreases angiogenic activity in AVM tissue, compared to that in untreated AVM tissue1). Moreover, in an experimental study by Jahan et al.9), SRS for an artificial animal AVM model showed a reduction in the size of the lesion, compared to that in the non-radiosurgical model. Although these studies are not results for SDAVF, we thought that it was possible to perform radiosurgery for SDAVF. Dalyai et al.4) reported that SRS for arteriovenous fistula lesions did not clearly show the mechanisms of treatment results, but they induced smooth muscle expansion, adventitial fibrosis, and an intimal response of arterial feeders, and eventually achieved obliteration of the fistula.
There are some points of note regarding SRS for SDAVF. It is necessary to include the arterialized fistula in the SRS target. However, it is not easy to find the exact location of fistulas with CT and MRI-based image-guided structural segmentation. Thus, we planned the target area to include the dura margin, because fistulas of SDAVF are located at the sleeve of the nerve root in the intradural space5131822). The planning of the radiosurgical dose was based some study, which the appearance of myelopathy from SRS to spinal lesions appear rare (<1%) when the maximum spinal cord dose is limited to the equivalent of 13 Gy in a single fraction or 20 Gy in three fractions1114). However, these studies did not provide sufficient long-term data, and our patient had preexisting myelopathy. Thus, we treated him with a slightly lower dose than those in the references.
CONCLUSION
Advanced imaging studies, including the three-dimensional sagittal TRICKS sequence, may be useful for obtaining information about SDAVF and in performing SRS and follow-up after SRS when selective spinal angiography has failed. And we think that SRS may be another treatment option, especially, for patients preferring non-invasive procedures. Our study has the obvious limitation of only including one treatment case, so additional cases of SDAVF treated by SRS are necessary to determine if it is an effective treatment for this condition.