Neural Growth Factor Stimulates Proliferation of Spinal Cord Derived-Neural Precursor/Stem Cells
Article information
Abstract
Objective
Recently, regenerative therapies have been used in clinical trials (heart, cartilage, skeletal). We don't make use of these treatments to spinal cord injury (SCI) patients yet, but regenerative therapies are rising interest in recent study about SCI. Neural precursor/stem cell (NPSC) proliferation is a significant event in functional recovery of the central nervous system (CNS). However, brain NPSCs and spinal cord NPSCs (SC-NPSCs) have many differences including gene expression and proliferation. The purpose of this study was to investigate the influence of neural growth factor (NGF) on the proliferation of SC-NPSCs.
Methods
NPSCs (2×104) were suspended in 100 µL of neurobasal medium containing NGF-7S (Sigma-Aldrich) and cultured in a 96-well plate for 12 days. NPSC proliferation was analyzed five times for either concentration of NGF (0.02 and 2 ng/mL). Sixteen rats after SCI were randomly allocated into two groups. In group 1 (SCI-vehicle group, n=8), animals received 1.0 mL of the saline vehicle solution. In group 2 (SCI-NGF group, n=8), the animals received single doses of NGF (Sigma-Aldrich). A dose of 0.02 ng/mL of NGF or normal saline as a vehicle control was intra-thecally injected daily at 24 hour intervals for 7 days. For Immunohistochemistry analysis, rats were sacrificed after one week and the spinal cords were obtained.
Results
The elevation of cell proliferation with 0.02 ng/mL NGF was significant (p<0.05) but was not significant for 2 ng/mL NGF. The optical density was increased in the NGF 0.02 ng/mL group compared to the control group and NGF 2 ng/mL groups. The density of nestin in the SCI-NGF group was significantly increased over the SCI-vehicle group (p<0.05). High power microscopy revealed that the density of nestin in the SCI-NGF group was significantly increased over the SCI-vehicle group.
Conclusion
SC-NPSC proliferation is an important pathway in the functional recovery of SCI. NGF enhances SC-NPSC proliferation in vitro and in vivo. NGF may be a useful option for treatment of SCI patients pending further studies to verify the clinical applicability.
INTRODUCTION
Traumatic spinal cord injury (SCI) is a serious and complex condition that causes severe and permanent dysfunction and disability. Research to improve and even reverse SCI damage has recently benefited from advancements in the knowledge of the pathophysiology, including the potential of stem cell therapy.
SCI pathophysiology comprises the direct, primary injury caused by the initial mechanical trauma and secondary injury due to a subsequent series of secondary degenerative processes that result in cell apoptosis. Primary injury is permanent, but although secondary injury should be preventable, no novel and efficient treatment options exist34262831).
Recently, regenerative therapies were used in clinical trials (heart, cartilage, skeletal)22936). We don't make use of these treatments to SCI patients yet, but regenerative therapies are rising interest in recent study about SCI.
Neural precursor/stem cells (NPSCs) are capable of dividing, migrating, and differentiating into neurons. NPSC proliferation is a significant event in functional recovery of the central nervous system (CNS)232437). Also, NPSC has been known to be effective for functional recovery in SCI according to the several previous studies172233). Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), insulin growth factor-1 (IGF-1), and basic fibroblast growth factor (bFGF) are essential neurotrophic factors involving in inducing the proliferation and differentiation of NPSCs18111237). It is known from previous studies that several neurotrophic factors affect the proliferation of spinal cord NPSCs (SC-NPSCs). In addition, there have been studies on the effect of NGF on brain NPSC5618). However, both brain NPSC and SC-NPSC have significant differences that include gene expression and proliferation1925). The purpose of this study, therefore, is to investigate the influence of NGF on the proliferation of SC-NPSCs.
MATERIALS AND METHODS
Animals
All animal experiments in this study involved adult male Sprague-Dawley (SD) rats weighing 290–310 g (Samtako Bio, Osan, Korea) that were cared for according to the national institutes of health guide for the care and use of laboratory animals. The animal resources and care committee of our university approved all surgical procedures. Animals were housed in a temperature controlled room using alternating 12-hour (hr) cycles of light and dark.
Culture of adult SC-NPSCs
Dissected rat spinal cord was chopped up using microscissors and incubated in a cocktail containing papain (0.1%, Worthington Biochemical Corp., Lakewood, NJ, USA), dispase (0.1%, Boehringer Mannheim, Mannheim, Germany), DNase (0.01%, Worthington Biochemical, Lakewood, NJ, USA), and MgSO4 (12.4%) in Hanks balanced salt solution (Invitrogen, Carlsbad, CA, USA) with glucose (0.45%) for 30 min at 37°C. The dissociated cells were cultured to form neurospheresin a neurobasal medium containing B-27 (Invitrogen, Carlsbad, CA, USA), glutamine (2 mmol/L), penicillin/streptomycin (0.1 g/mL, Invitrogen, Carlsbad, CA, USA), FGF-2 (20 ng/mL, R&D Systems, Minneapolis, MN, USA), and heparin (2 mg/mL, Sigma-Aldrich, St. Louis, MO, USA). After 7 days, the neurospheres were dissociated with Accutase (Innovative Cell Technologies, San Diego, CA, USA) and 5×105 NPSCs were grown in plates or dishes containing 5 mL of culture medium. For identify the NPSC gene, we performed the NPSC gene chip analysis in primary culture.
Cell proliferation assay
NPSCs (2×104) were suspended in 100 µL of neurobasal medium containing NGF-7S (Sigma-Aldrich, St. Louis, MO, USA) and cultured in a 96-well plate for 12 days. NGF concentration was 0.02 or 2 ng/mL. Culture medium was changed four times as once per 3 days. A 20-µL cell proliferation assay solution containing the tetrazolium compound MTS (CellTiter 96 Aqueous One Solution; Promega, Madison, WI, USA) was added to a well containing cultured cells and incubated for 2.5 hr at 37°C. The optical density was measured at 490 nm with a microplate spectrophotometer. The same volume of culture medium with the assay solution was used as a blank.
Spinal cord injury and administration of NGF
SD rats (n=16) were randomly allocated into two groups. In group 1 (SCI-vehicle, n=8), animals received 1.0 mL of the saline vehicle solution. In group 2 (SCI-NGF, n=8), the animals received single doses of NGF (Sigma-Aldrich, St. Louis, MO, USA). Rats were anesthetized intra-peritoneally with a mixture of xylazine (10 mg/kg) and ketamine (60 mg/kg). After laminectomy at T9–10, a modified aneurysm clip with a closing force of 30 grams (Aesculap, Tuttlingen, Germany) was held in an applicator. The clip applied vertically to the exposed spinal cord for a 2 minutes compression. Bladders were manually emptied twice daily until spontaneous voiding occurred. A dose of 0.02 ng/mL of NGF or normal saline as a vehicle control was intra-thecally injected daily at 24 hr intervals for 7 days.
Immunohistochemistry of nestin
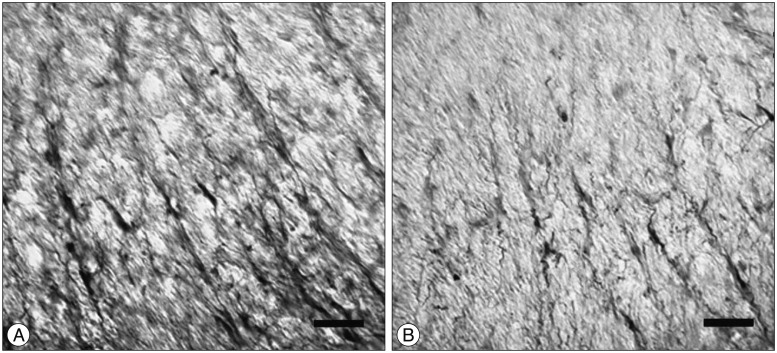
For immunohistochemistry (IHC) analysis, rats were sacrificed after one week and the spinal cords were obtained. Spinal cord tissue was sectioned at a thickness of 30 µm on a cryostat and sections were floated on the surface of 0.1 M phosphate buffer. To detect nestin (marker for neural stem cells), 5- and 6-mm sections of rostral spinal cord were selected. The sections were blocked with 4% normal serum in 0.5% Triton X-100 for 1 hr at room temperature and incubated over night at 4°C with a 1 : 2000 dilution of mouse monoclonal anti-nestin (R&D Systems, Minneapolis, MN, USA). Sections were incubated in 0.1 M phosphate buffer containing 4% normal serum and 0.5% Triton X-100 for 2 hr at 25°C on a shaker, and then in primary antiserum in 0.1 M phosphate buffer containing 4% normal serum and 0.5% Triton X-100 for 12 hr at 25°C. After rinsing (3×10 min) in 0.1 M phosphate buffer, sections were exposed to a 1 : 200 dilution of biotinylated anti-mouse IgG (Sigma-Aldrich, St. Louis, MO, USA) in 0.1 M phosphate buffer containing 4% normal serum and 0.5% Triton X-100 at 25°C for 2 hr. The sections were then incubated in a 1 : 50 dilution of avidin-biotinylated horseradish peroxidase (Vector Laboratory, Burlingame, CA, USA) in 0.1 M phosphate buffer for 2 hr and rinsed (3×10 min) in 0.25 M Tris. Staining was visualized by reaction with 3, 3'-diaminobenzidine tetrahydrochloride (DAB) and hydrogen peroxide in 0.25 M Tris for 3–10 minutes using a DAB reagent set (Kierkegaard & Perry, Gaithersburg, MD, USA). All sections were washed out in 0.1 M phosphate buffer and mounted on Superfrost Plus slides (Fisher, Pittsburgh, PA, USA). Sections were dried overnight at 37°C. The tissues were photographed using a Zeiss Axiopan microscope with high power DIC optics (Carl Zeiss, Jena, Germany). The images were viewed on a computer monitor using a Zeiss Plan-Apochromat 40× objective (Carl Zeiss, Jena, Germany) and photographs of the ventral side of white matter of both sides were taken with a Zeiss Axio Cam HRc digital camera (Carl Zeiss, Jena, Germany). Enumeration of immune-positive cells was done using a Lab works version 4.5 computer-assisted image analyzer (UVP, Upland, CA, USA).
Statistical analysis
All statistical comparisons were computed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as mean±standard error (SE) of the means. Repeated measure ANOVA was used to compare groups. Null hypotheses of no difference were rejected if p-values were <0.05.
RESULTS
NGF affects SC-NPSC proliferation in vitro
NGF concentrations of 0.02 and 2 ng/mL were used. NPSC proliferation was assayed five times for either concentration. When compared to the control group, NPSC proliferation was increased at both concentrations. The average cell proliferation was 0.39646 (optical density ratio 100) in the control group, 0.4295 (optical density ratio 108.3) in the 0.02 ng/mL NGF group, and 0.41478 (optical density ratio 104.6) in the 2 ng/mL NGF group. The elevation of cell proliferation with 0.02 ng/mL NGF was significant (p<0.05), but was not significant for 2 ng/mL NGF (Fig. 1). The optical density was increased in the NGF 0.02 ng/mL group compared to the control and NGF 2 ng/mL groups (Fig. 2).
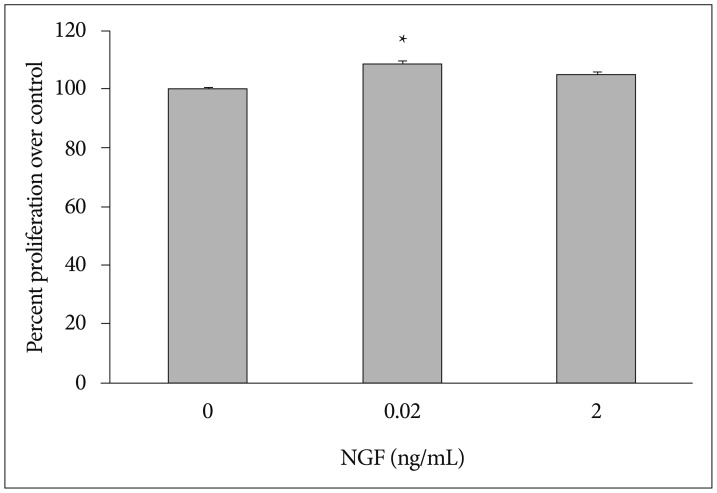
Optical density ratio in the lower concentration of NGF 0.02 group (*) was 108.3 (average of five independent determinations; p=0.0007). NPSC proliferation was significantly increased in the NGF 0.02 group (*) compared to the control group. NGF : nerve growth factor, NPSC : neural precursor/stem cells.
NGF induces the proliferation of NPSCs after SCI
SCI in rats was followed by an intra-thecal injection of 0.02 ng/mL of NGF or saline (n=8/group). The injections were done at 24-hr intervals for 7 days. One week after SCI, the injured rats were sacrificed, spinal cords were collected, and prepared sections of white matter of the ventral side of each spinal cord were analyzed for nestin immune reactivity. ANOVA was used to confirm density areas of nestin. The expression of nestin in the SCI-NGF group was significantly increased over the SCI-vehicle group (p<0.05) (Fig. 3). High power microscopy revealed that the density of nestin in the SCI-NGF group was significantly increased over the SCI-vehicle group (Fig. 4).
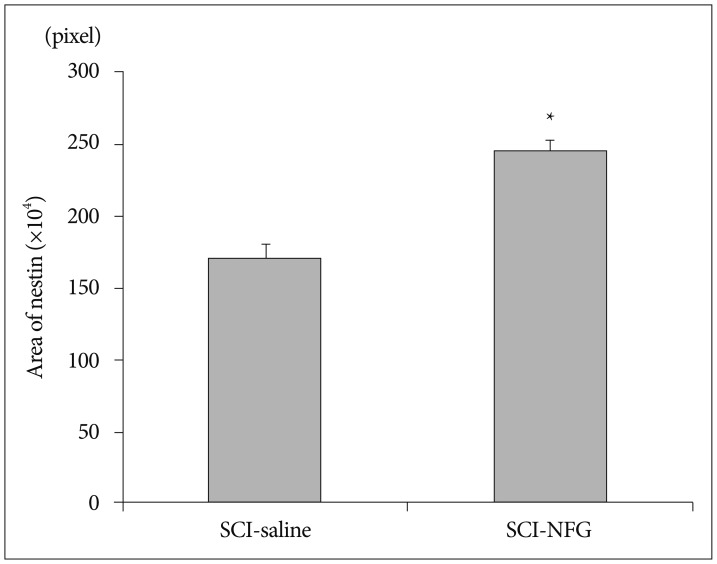
The expression of nestin at the cord ventral portion in the SCI-NGF group (*) was significantly increased when compared to the SCI-vehicle group. SCI : spinal cord injury, NGF : nerve growth factor.
DISCUSSION
Traumatic SCI causes irreversible neurological deficits in motor and sensory systems35). Patients with traumatic SCI are at great risk of substantial morbidity and mortality. Many pathological changes after a SCI are secondary to the initial impact, and are active biological processes. To overcome permanent damage after SCI, it is important to discontinue the secondary injuries and restore the damaged spinal cord neural networks. While stem cell therapy is theoretically possible, restoration of an impaired neural network can be technically elaborate and difficult15162032). NPSC proliferation after SCI is important in the recovery from neurological dysfunctions. The proliferated cells can differentiate to neurons, astrocytes, and oligodendrocytes. Also, NPSCs improve angiogenesis that enable endogenous repair and axonal outgrowth101722).
It is well known that NGF can promote brain NPSC proliferation5618). However, brain NSPCs and SC-NPSCs are markedly different, such as in gene expression and cell proliferation1925). NGF effect on SC-NPSCs is unclear in spinal cord, reflecting the different pathology of SCI from brain injury.
In present study, NGF affected proliferation of SC-NPSCs. In vitro, SC-NPSC proliferation was increased in a NGF dose-related fashion compared to the control group. SC-NSPCs was significantly enhanced in the presence of 0.02 ng/mL NGF, but was not 2 ng/mL NGF. Adverse effects, such as aberrant sympathetic and sensory neurite sprouting2739), and weight loss38) are observed in previous studies on brain NGF. Also, it is claimed that there is an optimal dose with a maximal effect of NGF although slight difference exists in accordance with used animals and types of tissues (brain, spinal cord, etc.)273839). In this study, we evaluated only two concentrations of NGF (0.02 and 2 ng/mL) and we cannot be sure that this result is most accurate value. However, 0.02 ng/mL of NGF is statistically significant value in vitro study, and we used this concentration of NGF in vivo study.
Intra-thecal injection of NGF to SCI rats increased the density of nestin on the cord ventral portion. This result is consistent with the in vitro findings. NGF promotes NPSC proliferation in brain injury7930). NGF is the first and most comprehensively studied neurotrophic factor. In vitro, NGF can induce various neural stem cells, embryonic stem cells, and mesenchymal stem cells from different species to differentiate into specific neurons1440). The present results reveal the same effect in spinal cord.
There are several limitations of our study. First, neural differentiation was not evaluated in this study, but NGF is mainly correlated proliferation of NPSC5618). So this study focused on NPSC proliferation in vitro and in vivo. Second, SCI rats were injected intra-thecally. This is an invasive procedure that can directly injure the spinal cord. However, NGF does not pass the blood-brain barrier232434). Thus, intra-thecal injection is necessary to evaluate the influence of NGF effect on spinal cord. The method has been established for SCI model1321). Third, we did not examine functional recovery. It has been known from previous studies that NPSC is helpful for functional recovery in CNS232437). When in this study the fact is taken into consideration that NGF stimulates the proliferation of SC-NPSC, it is assumed that NGF contributes to functional recovery in SCI. However, further studies are required to identify the clinical effect of NGF in the future.
CONCLUSION
SC-NPSC is an important pathway in the functional recovery of SCI. NGF enhances SC-NPSC proliferation in vitro and in vivo. NGF may be a useful option for treatment of SCI patients pending further studies to verify the clinical applicability.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A01056299).