INTRODUCTION
Chronic hypoventilation due to dysfunction of the brain stem or the high cervical spinal cord poses a serious medical and social problem12). Patients with chronic ventilatory failure are managed with the use of artificial ventilators. However, chronic use of positive pressure ventilation is not physiological, easily causes infections, and restricts the patient's activities. For example, the rehabilitation and ambulation of the quadriplegic patient who is completely dependent on a positive pressure respirator is a serious burden on both the patient and family and the emotional adjustment is difficult for the younger patients6). The premise that electrical stimulation of the phrenic nerve could be used to induce diaphragmatic contraction and artificial ventilation dates back to the late 1700s; Cavallo recommended the use of electricity for human ailments in 19773). Long-term continuous pacing of the conditioned diaphragm as it exists today became possible through the pioneering efforts of Judson and Glenn8). Almost all patients with diaphragm pacing so far have been using a device specifically made for this purpose by Avery Laboratories Inc. (Commack, NY, USA) or Atrotech OY (Tampere, Finland)5-8). However, these devices are not available in eastern Asian countries. Taira et al.12) proposed an alternative use of electrical stimulators used in spinal cord stimulation and deep brain stimulation in patients with chronic ventilatory failure who were dependent on respirator. We report our experience of an application of spinal cord stimulator for diaphragm pacing in a quadriplegic patient with central apnea due to high cervical cord injury.
CASE REPORT
Clinical presentation
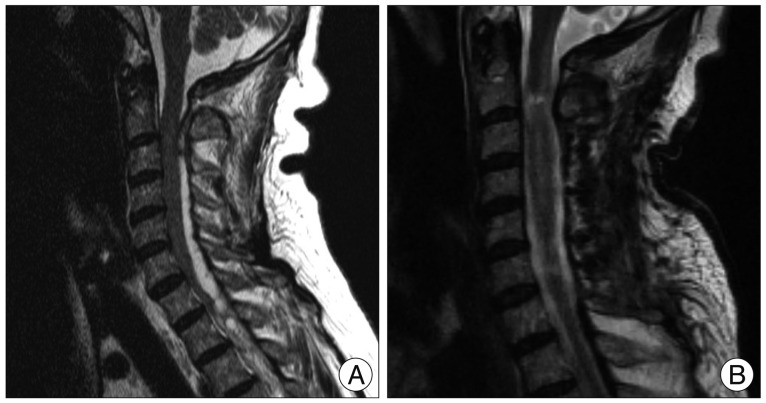
A 62-year-old female patient reported severe dyspnea and showed tachypnea. She was diagnosed as a pulmonary thromboembolism and immediate anticoagulation with warfarin was started. In the morning two days after anticoagulation, she felt heaviness in both legs and progressive paraparesis. A neurologist evaluated her and requested a lumbosacral magnetic resonance imaging (MRI) of the lumbar spine. While waiting for the MRI, her paraparesis progressively increased and ascended; eventually she became quadriplegic and respiratory distress developed. After checking whole spine MRI, an emergent endotracheal intubation was performed and neurosurgical consultation was taken. On examination, she was alert and no focal cranial nerve sign was observed. However, grade 0 quadriplegia was noticed and tendon reflex was not elicited. An acute epidural hematoma was noted on the high cervical cord (Fig. 1A). After informed consent, an emergent decompression of the cervical cord was performed. Unfortunately, her quadriplegia did not improve after surgery. Moreover, self-respiration was not elicited. Thereafter, she was maintained with artificial ventilation during the following three months in the intensive care unit. During the follow-up, no spontaneous, voluntary respiration was observed and the MRI checked at postoperative 3 months showed a myelomalacia at the C2 level suggesting high cervical cord injury (Fig. 1B). A standard apnea test did not elicit voluntary respiratory effort. After 3 months of artificial ventilation, we decided to perform a trial of diaphragm pacing, and informed consent was taken. An electromyographic examination of the diaphragm was performed for the verification of contraction of the diaphragm.
Operative procedure
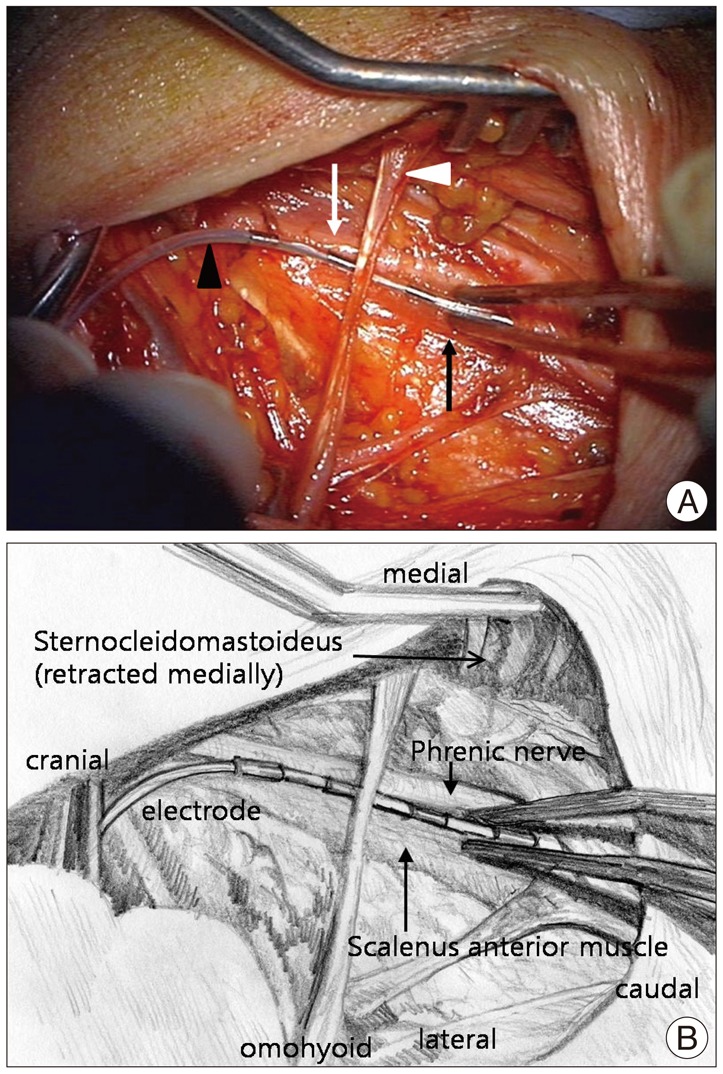
A stimulation lead implantation was performed in right neck under general anesthesia with the head rotated to the left side. Muscle relaxant was not used to monitor intraoperative contraction of the diaphragm. A linear horizontal skin incision about 4 cm was made at 4 cm rostral to the right clavicle, crossing the posterior border of the sternocleidomastoideus muscle. After dissecting the subcutaneous tissue and retracting the sternocleidomastoideus medially, the anterior scalene muscle and the right phrenic nerve was identified over the scalene muscle with monopolar electrical stimulation (50 Hz, 1 msec, 1.5-2 volts, Eclipse Neurological Workstation, Axon Systems, USA) (Fig. 2A). The nerve sheath of the phrenic nerve was carefully dissected about 3 cm in length, but we did not dissect the space between the nerve and the scalene muscle. A quadripolar, cylindrical electrode (QuatrodeВ®, St. Jude Medical, Plano, TX, USA) was placed along the phrenic nerve and fixed with 3 sutures to the surrounding connective tissue (Fig. 2B). A great care was taken to avoid injury and entrapment of the phrenic nerve. During intraoperative stimulation, a brisk downward deflection of the diaphragm was noticed under fluoroscopy. The distal end of the electrode was tunneled subcutaneously to the left subclavicular subcutaneous pocket and connected to the implantable pulse generator (IPG) (Eon-Mini RechargeableВ®, St. Jude Medical, Plano, TX, USA).
Postoperative course
Two days after surgery, the IPG was turned on. The second proximal contact of the quadripolar electrode was used as cathode and the third contact was used as positive. The other contacts were set to "off". After turning the IPG on, the carbon dioxide pressure of the exhalation and the arterial oxygen saturation in the fingertip were monitored until the values became stable. The carbon dioxide pressure in the end tidal breath was adjusted to about 400 mm Hg by changing the output strength of the IPG. The parameters of chronic stimulation were 1(-), 2(+), 300 Вөsec, 30 Hz, 2 seconds on with 4 seconds off in cyclic mode. After confirmation of stable end-tidal PCO2 and SaO2 levels for more than two hours in the patient's bed, the patient was allowed to ambulate in a wheelchair without mechanical ventilation. The ambulation time was gradually increased up to two hours. However, the weaning time of mechanical ventilation could not be increased more than three hours due to fatigue of breathing chest muscles. She was weaned and could ambulate in the wheelchair for two to three hours in daytime, spending time with her family, and she was dependent on the mechanical ventilator for the rest of the time. At an examination twelve months after the operation, she could ambulate with diaphragm pacing in the wheelchair for two to three hours in daytime. Mild neck discomfort around the electrode implantation site was noticed; however, there was no evidence of granulation or infection during the follow-up. She suffered pneumonia two times after diaphragmatic pacing which was easily controlled with antibiotics during the follow-up.
DISCUSSION
Diaphragmatic pacing
The history of diaphragm pacing by phrenic nerve stimulation dates back to 1786; Caldani10) noted movement of the diaphragm upon electrical stimulation of the phrenic nerve. In 1873, Hefeland10) proposed stimulating the phrenic nerve to treat asphyxia neonatorum. Despite its documented success in the resuscitative process, stimulation of the diaphragm to activate the diaphragm was discontinued as a method of artificial ventilation became available. The work of Sarnoff4) and his associates was particularly important in the history of diaphragm pacing. They showed that adequate alveolar ventilation, so-called "electrophrenic respiration", could be maintained for at least 52 hours by transcutaneous stimulation of one phrenic nerve in the neck. Long-term, continuous pacing of the conditioned diaphragm as it exists today became possible through the pioneering effort of Glenn et al.6,7). In their report in 1985, 77 patients were treated by diaphragm pacing, followed for at least 5 years; 33 (52%) were paced for 5 to 10 years, and 15 (24%) were paced for 10 to 16 years10). Thus, long-term stimulation of the phrenic nerves to pace the diaphragm is an effective method of ventilator support in selected cases.
Diaphragm pacing is conducted with low frequency electrical stimulation at a slow repetition (respiratory) rate to condition the diaphragm muscle against fatigue and maintain it fatigue-free6). This means that diaphragmatic conditioning is a process by which the fast-contracting fibers (anaerobic, highly glycolytic fibers), which are highly susceptible to get fatigued, are converted to slow-conducting fatigue-resistant fibers (aerobic oxidative fibers), which enable permanent ventilator support using diaphragmatic pacing4,5). Clinical observations of the tolerance of diaphragmatic pacing and spirometric findings during the conditioning period and over more prolonged periods allowed diaphragmatic muscle fatigue to be excluded1).
Candidates for diaphragm pacing are those with ventilator insufficiency due to malfunction of the respiratory control center in the brain stem (central alveolar hypoventilation) or interruption of the upper motor neurons of the phrenic nerve6). Diseases which destroy or disrupt the upper motor neurons of the phrenic nerve in the brain, such as stroke, tumor, infection, and trauma in the medulla, are amenable to phrenic nerve pacing if the viability and function of the phrenic nerve and diaphragm are proved to be intact. Those patients whose cervical injury is above the C3 level are most likely to benefit from pacing because the nerve roots of C3 to C5 maintain complete integrity of the phrenic nerve proper3). Patients whose cord injury involves any part of the C3 to C5 nerve roots have injury to the phrenic nerve itself, which may interfere with pacing. Those patients with cord injury below the C5 level have an intact and functional phrenic nerve and should be able to respire independently, although this may not be apparent at the time of injury. In this circumstance, a waiting period of approximately 3 months may be needed to delineate the degree of dysfunction more clearly3). Phrenic nerve stimulation serves no purpose in patients without an intact phrenic nerve and functional diaphragm. Accurate assessment of both components is imperative. The viability of the phrenic nerve was explored preoperatively by measuring conduction time using the Davis2) and Shaw et al.11) technique consisting of transcutaneous stimulation of the phrenic nerve in the neck. Conduction times of 4-12 msec was considered normal; patients having longer conduction times were excluded from surgery. The response to electrical stimulation was evaluated by fluoroscopic or echographic study of diaphragmatic mobility.
Application of spinal cord stimulator system for diaphragm pacing
The reason we started using the spinal cord stimulator for pain control instead of the commercially available system (Avery laboratories Inc.) was that this equipment was not available in our country and we think this situation is true in many other countries. Furthermore, the feasibility of application of the spinal cord stimulator and brain stimulator in diaphragm pacing has already been reported where the commercial system also was not available13). Indeed, Taira et al.12) reported successful application of spinal cord or deep brain stimulators for phrenic nerve stimulation to pace the diaphragm in four patients with chronic hypoventilation. They demonstrated the effectiveness and safety of unilateral (right-sided) phrenic nerve stimulation in patients completely dependent on mechanical ventilation for central apnea due to brain stem injuries.
Diaphragm pacing through implanted electrodes system has been performed through trans-thoracic and cervical approaches4,12,13). Placement of the electrodes for the Avery or Atrotech model is carried out through a limited anterior thoracotomy in the second or third intercostal space. Alternatively, the electrode can be placed via a cervical approach for its technical simplicity, theoretically lower morbidity, and feasibility of bilateral implantation4). However, some authors advocated trans-thoracic approach because of the presence of the accessory phrenic nerve that does not join the main phrenic nerve trunk until the mediastinum and anatomic variation in the course of the phrenic nerve in the anterior scalene space3). For the placement of the electrode for spinal cord stimulator for diaphragm pacing, the cervical approach was advocated by Taira et al.12).
In the present case of central apnea from high cervical cord injury, we adopted the right-sided cervical approach using spinal cord stimulator for diaphragm pacing proposed by Taira et al.9,12,13). However, the application of spinal cord stimulator in this particular patient, central apnea from cervical epidural hematoma, is still extremely rare and this has not been reported so far. Indeed, there were only two cases of application of spinal cord stimulator in the high cervical cord injury in the literature; apnea from C1-2 cord gunshot injury and surgical complication of atlantoaxial dislocation9,13). Diaphragm pacing with the spinal cord stimulator is feasible and effective for the treatment of the central hypoventilation syndrome.