Lee, Lee, Kim, Lee, Kim, and Kim: A Role of Serum-Based Neuronal and Glial Markers as Potential Predictors for Distinguishing Severity and Related Outcomes in Traumatic Brain Injury
Abstract
Objective
Optimal treatment decision and estimation of the prognosis in traumatic brain injury (TBI) is currently based on demographic and clinical predictors. But sometimes, there are limitations in these factors. In this study, we analyzed three central nervous system biomarkers in TBI patients, will discuss the roles and clinical applications of biomarkers in TBI.
Methods
From July on 2013 to August on 2014, a total of 45 patients were included. The serum was obtained at the time of hospital admission, and biomarkers were extracted with centrifugal process. It was analyzed for the level of S-100 beta (S100B), glial fibrillary acidic protein (GFAP), and ubiquitin carboxy-terminal hydrolase-L1 (UCH-L1).
Results
This study included 33 males and 12 females with a mean age of 58.5 (19-84) years. TBI patients were classified into two groups. Group A was severe TBI with Glasgow Coma Scale (GCS) score 3-5 and Group B was mild TBI with GCS score 13-15. The median serum concentration of S100B, GFAP, and UCH-L1 in severe TBI were raised 5.1 fold, 5.5 fold, and 439.1 fold compared to mild injury, respectively. The serum levels of these markers correlated significantly with the injury severity and clinical outcome (p<0.001). Increased level of markers was strongly predicted poor outcomes.
Conclusion
S100B, GFAP, and UCH-L1 serum level of were significantly increased in TBI according to severity and associated clinical outcomes. Biomarkers have potential utility as diagnostic, prognostic, and therapeutic adjuncts in the setting of TBI.
Key Words: Brain injuries · Biological markers · S100 Calcium Binding Protein beta Subunit · Glial fibrillary acidic protein · Ubiquitin thiolesterase.
INTRODUCTION
In recent years, traumatic brain injury (TBI) is not only simple phenomenon confined to damage injured by, but also considered heterogeneous pathological disease state affecting all ages. TBI has a peak incidence between 15-24 years of age 10,25). Since it is prevalent in children and young adults, the result of TBI has a serious and devastating effect in social and economic terms 23). Injury grading is very various, range from mild with low mortality rate to severe with life-threatening lesion. The mainstay treatment for TBI is to reduce the extent of secondary brain damage after the primary insult 23). Prediction of the severity and prognosis in TBI is currently based on demographic, clinical and radiological features, including age, initial Glasgow Coma Scale (GCS) score, pupillary response, vital signs, significant non-cranial injuries, and computed tomography (CT) indices. The clinical outcome prediction by those informations have several limitations because neurologic assessment is often performed not exactly by the use of many drugs (analgesics, sedatives, neuromuscular blocking agent, etc.) and sometimes, discrepancy exists when compared initial radiological information with clinical course, especially in diffuse injury without focal mass lesion. Therefore, there is enormous diagnostic and prognostic promise in developing assays that measure TBI-associated biomarkers accurately and specifically 36). A biomarker is an indicator of a specific biological or disease state that can be measured using samples taken from either the affected tissue or peripheral body fluids. In other organ disease and injury, the specific-markers that have proven invaluable are actively applicated in clinical field as rapid diagnostic tools. But in TBI, there is no such definitive biomarker. Previously investigated markers in large studies include neuron-specific enolase (NSE) 27,33), myelin basic protein 5,33), glial protein S-100 beta (S100B) 18,2431), glial fibrillary acidic protein (GFAP) 16). Recently, as a result of increased development of proteomics analysis and other discovery techniques, investigators have reported ubiquitin C-terminal hydrolase-L1 (UCH-L1), neuron specific and concentrated in neuronal soma, as a novel potential marker for brain injury 13). We investigated the levels of S100B, GFAP, and UCH-L1 in peripheral blood, and would determine the predictive value of the serum level of these markers for clinical outcomes after TBI. In addition, we hypothesized that the combination analysis of neuronal cell body marker (UCH-L1) and glial markers (S100B, GFAP) could reflect different pathophysiology would be more informative than either biomarker alone or only glial and neuronal same category markers investigation.
MATERIALS AND METHODS
From July on 2013 to August on 2014, we investigated 45 patients with TBI. On admission, CT scans were performed in all patients. The following variables were recorded for each of the patients; age, sex, a history of specific diseases, kind of trauma, the time of hospital admission, radiologic findings, initial GCS score, status of pupils, treatment modality, and GCS score at discharge. The patients were classified into two groups according to clinical manifestations.
1) The group A, termed the "severe injured group", consisted of those who had GCS score under 6 on admission
2) The group B, consisted of those who had GCS score of 13 to 15 on admission
In all enrolled patients, initial blood sampling was performed within 24 hours after trauma. The routine blood examinations were performed via peripheral veins. The first blood sample was obtained within 4 hours arrival on hospital. We investigated three makers in this study : 2 glial markers (S100B, GFAP) and 1 neuronal maker (UCH-L1).
Biomarker identification
To determine the level of S100B, GFAP, UCH-L1 protein, blood samples were centrifuged, separated and serum was stored at -50℃ until the analysis, and once gathered, serum samples from all patients were analyzed by sandwich enzyme-linked immunosorbent assay (ELISA) to S100B, GFAP, and UCH-L1 with a high degree of sensitivity using a commercially available kit. Monoclonal antibody specific for S100B has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any S100B present is bound by the immobilized antibody. A biotinylated polyclonal antibody specific for S100B is added to the wells. Following a wash to remove any unbound reagent, an enzyme complex is added to the wells. After incubation and washing, a substrate solution is added to the wells. The GFAP ELISA utilized a proprietary mouse monoclonal antibody for solid phase immobilization and a proprietary polyclonal rabbit antibody for detection. The antibodies detect both whole GFAP molecules as well as GFAP breakdown products, potentially resulting in a more complete measure of GFAP levels in circulation 36). The UCH-L1 was assayed using a sandwich ELISA as previously described 22). Reaction wells were coated with mouse monoclonal antibody, capture antibody in 0.1 M sodium bicarbonate, pH 9 and incubated overnight at 4℃. Plates were then emptied out and blocking buffer was added and incubated for 30 minutes at ambient temperature with gentle shaking. This was followed by addition of antigen standard. The plate was incubated for two hours at room temperature then washed using an automatic plate washer. Rabbit polyclonal antibody, detection antibody in blocking buffer was then added to wells at 100 µL/well and the plates were further incubated for 1.5 hours at room temperature. After additional automatic washing, biotinyl-tyramide solution was added and the plate was incubated for 15 minutes at room temperature followed by automatic washing. Addition of streptavidin-horseradish peroxidase (1 : 500, 100 uL/well) and 1% bovine serum albumin for 30 minutes incubation at room temperature was followed by automatic washing. Finally, the wells were developed with substrate solution : Ultra-Trail du Mont-Blanc ELISA (100 uL/well) with incubation for 5 to 30 minutes and read at 652 nm with spectrophotometer. We compared serum levels of S100B, GFAP, and UCH-L1 in severe (GCS score<6) and mild injured group (GCS score 13 to 15) at each of the admission time. Acute injury severity was functionally assessed by the GCS score on admission. If there was difference in GCS score between admission and a few hours after hospitalization, the 24-hour post injury GCS score was used as an end point because the GCS score of a few patients was confused by other factors, including the presence of alcohol, analgesics, sedatives in the first several hours after injury. We also compared biomarker levels according to CT findings reveal parenchymal injury or not. Clinical outcome after acute injury was assessed by the Glasgow Outcome Scale score at 3 months after TBI.
The Mann-Whitney test was used to determine the difference of biomarker serum level between both groups in glial and neuronal proteins. In all investigated cases, statistical significance was established in p<0.05. Spearman rank correlation test was used to test correlations between quantitative variables for significance. The receiver operator characteristics (ROC) curve was used to assess the extent to which the S100B, GFAP, and UCH-L1 levels differed between both groups with good recovery after minor injury. The ROC curve was obtained by calculating the sensitivity and specificity for every distinct observed data value, and plotting sensitivity against 1-specificity. Diagnostic accuracy was assessed by the area under the curve (AUC). This area represents the probability that a person, with disability after head injury has a higher S100B, GFAP, and UCH-L1 level than head injured person without disability. The ROC curve was used to determine cutoff value that insured a high proportion of patients with poor outcomes after TBI would be detected (high sensitivity) at the cost of a relatively high false positive rate (low specificity). Analysis was performed using the Statistical Package for Social Sciences (SPSS 11 for windows; SPSS Inc., Chicago, IL, USA).
RESULTS
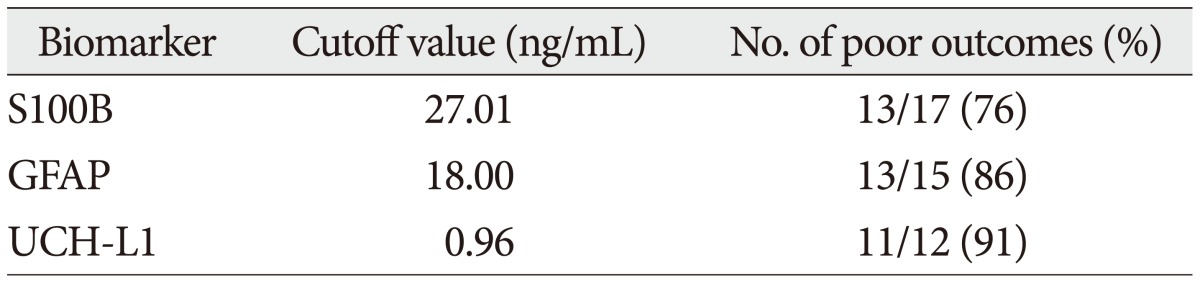
The demographic characteristics and neurologic data are summarized as follows ( Table 1). In 45 patients that were enrolled in this study, 33 patients were male and 12 patients were female. The mean age was 58.5 years, ranged from 19 to 84. The patients were classified into two groups. The group A included 14 patients, and the group B included 31 patients. The mean initial GCS score was 5.3 and 14.5 in group A and B. In the CT scan, the parenchymal injury was shown in 20 patients and 25 patients had non parenchymal injury. The patients with severe TBI had significantly elevated serum concentration of S100B, GFAP, and UCH-L1 after injury compared with minor injured group. As expected, S100B and GFAP showed inverse correlation between GCS score and serum level, but there was no such pattern in UCH-L1. The UCH-L1 frequently was not detected in minor injured group. Although detected in minor injured group, the concentration of serum level was ranged from 0.07 to 1.45 ng/mL. In severe injured group, the concentration was ranged from 0.96 to 151.25 ng/mL. Minor injured patients had relatively very low serum concentration compared to severe group ( Fig. 1). Four patients were dead during hospitalization and the others had poor clinical outcome in severe injured patients (group A, n=14). All of minor injured patients (group B, n=31) had good clinical course ( Table 2). The median serum concentrations of markers taken at the time of hospital admission in patients with severe TBI were raised 5.1 fold for S100B, 5.5 fold for GFAP, and 439.1 fold for UCH-L1 compared to the mild injury group. Furthermore, the median serum S100B, GFAP, and UCH-L1 concentration were higher in patients who were dead compared to survival patients post TBI. The increasing ratio of serum concentration was similar in S100B and GFAP, but in UCH-L1, there was enormous difference between severe and mild injured group. The statistical significant elevation of each markers was found in severe injured and dead patients ( p<0.001; Mann-Whitney test Table 3, Fig. 2; Table 4, Fig. 3). It suggests S100B, GFAP, and UCH-L1 can be significant predictor for severity and clinical outcome in TBI. The patients who had parenchymal injury on CT scan had higher serum concentrations of S100B, GFAP, and UCH-L1 than the patients without parenchymal injury. Similarly, when analyzed within each group, the patient with parenchymal injury on CT scan was higher than non injured group ( Table 5). The correlation between the brain specific markers and clinical indices, demographic characteristics and neurologic data were as follows ( Table 6). The brain specific proteins were seems to be mildly inter-correlated with demographic characteristics. Age and sex were not shown significant correlation to any markers. The statistical significant correlation was presented between initial GCS score reflecting injury severity and all markers ( p<0.01). Only GFAP had significant correlation parenchymal injury on CT scan, not to S100B and UCH-L1 ( p<0.01). Because GFAP is specific glial protein as compared with neuronal specific UCH-L1, it more reflect to general damage to direct injury. In analysis of each markers, glial proteins (S100B and GFAP) were significantly correlated with each other. But there is no statistical significant correlation between glial (S100B and GFAP) and neuronal marker (UCH-L1) ( Fig. 4). The ROC curves of the S100B, GFAP, and UCH-L1 was created to explore the ability sensitivity and specificity of prediction of poor outcome ( Fig. 5). The AUC was 0.95 for S100B, 0.99 for GFAP, and 0.88 for UCH-L1. The figure clearly shows higher AUC for S100B, GFAP, and UCH-L1. The result of this definite curve might be influence from the relatively small number of patients enrolled in this study and exclusion of moderate injured patients. The cutoff values were established 27.01, 18.00, and 0.96 ng/mL in S100B, GFAP, and UCH-L1 as the best operating point. To predict of poor outcomes by these values, high prediction rate was achieved ( Table 7). The sensitivity and specificity of this cutoff value for poor outcome is 92%, 87% in S100B, 92%, 93% in GFAP, and 78%, 96% in UCH-L1.
DISCUSSION
A biomarker is a biological indicator of a specific or pathological state. Molecular biomarkers can take many forms and, as a consequence, a variety of strategies have been undertaken for their discovery, including transcriptional, proteomic, and metabolomic profiling. Molecular markers have been detected in tissue samples or body fluids. For example, the release patterns the MB isozyme of creatine kinase (CK-MB, predominantly found in cardiac muscle) following myocardial infarction provides information related to the size of the infarction, the effect of treatment, and the prognosis of the patient 9). Troponin levels have also become a standard biomarker used in the diagnosis and determination of treatment for myocardial infarction 1,14). The identification pathology-specific biomarkers can assist in the diagnosis and estimation of prognosis, and can serve as surrogate markers for monitoring the effectiveness of a treatment. Our prospective observational study in 45 patients with severe or mild TBI demonstrates that prediction of severity of brain damage and clinical outcome after TBI may be improved with the determination of serum level of brain specific proteins (S100B, GFAP, and UCH-L1) at the time of hospital admission. The analysis of brains specific proteins S100B, GFAP, and UCH-L1 together with GCS score can be used best predictive tool for severity and outcome in TBI patients. Our data is in line with previous study that showed a correlation of S100B, GFAP, and UCH-L1 with mortality after severe TBI 7,2633). The S100B and NSE serum concentration were higher in the first hours post injury in patients with poor compared to good outcome 19,32). The relevance of the determination of S100B protein in serum is not only restricted to severe TBI. After minor head injury raised S100B serum levels within 12 hours post injury predict neuropsychological dysfunction on measures of reaction time, attention, and speed of information processing after 12 months 3). The cerebral origin of S100B in peripheral blood is not always obvious. S100B is expressed not only in brain tissue but also in white and brown fat, skin, and skeletal muscle tissue (in much lower concentrations than in the brain 29,35)). Increased serum S100B levels after heart surgery have been shown to be at least partly of extra-cerebral origin, probably as a result of surgically traumatized fat, muscle and bone marrow. Also, in multi-trauma patients, extra-cerebral S100B may contribute to the measured serum level 2). The uncertainty in the cerebral or extracerebral origin of serum S100B probably does not exist for GFAP and UCH-L1. GFAP is an intermediate filament monomer found only in the cytoskeleton of astroglial cells, and thus is specific for the central nervous system (CNS). Therefore it is likely that serum GFAP is entirely derived from the brain. UCH-L1, also known as neuronal specific gene product 9.5, is the most strongly associated with the CNS, where it is predominantly detectable in neuronal cell bodies. Previous studies have identified inflammatory response molecules, excitotoxic amino acids, oxidative stress markers, and enzymes in cerebrospinal fluid (CSF) 4,811,1721,34). But measurement of CSF biomarkers requires placement of ventricular catheters, which may influence CSF protein levels 6,15) and may leading to complication such as infection. If it is possible to extract biomarkers from peripheral blood, more convenient access to study with low complication will be achieved. The UCH-L1 level was also examined in only CSF as a biomarker of severe TBI 12,28). Siman et al. 30), also reported an elevation of UCH-L1 signals, among a panel of other putative markers, in CSF of severe TBI patients. Mondello et al. 20), were the first to systematically assess UCH-L1 in human serum after TBI and to compare levels with those found in CSF of the same patients. They confirmed that UCH-L1 protein is present in human serum and that its levels are significantly elevated after severe TBI using ELISA analysis. Additionally, UCH-L1 was detectable in blood very early after injury, related to injury magnitude and may be an early predictor of clinical outcome. In our study, not all, UCH-L1 was detected in serum of mainly severe injured group and levels of UCH-L1 were significantly elevated in serum from severe injured subjects compared to mild group. This result maybe imply that because UCH-L1 is very specific proteins reflecting neuronal cell body injury, although the astrocyte or other glial component were damaged, if damage of neuronal cell body was not severe, significant value is not detected. For this reason, the polarization might be seen in scatter plot of UCH-L1. The pathophysiology pattern could be various in TBI. Although glial tissue damage exists, the neuronal cell body always is not damaged in TBI and less vulnerable to injury than glial cells. Glial cells are more affected to injury than neuronal cell and according to severity of injury, damage patterns could be difference. Though the biomarkers were investigated in past, single marker, only each glial markers, glial and neuronal marker reflecting axonal injury, not neuronal cell body marker were analyzed 29,35). For this reason, multi-markers analysis that presents glial and neuronal cell body damage should be performed. A single biomarker or same category markers may not have the desired level of sensitivity and required specificity for diagnostic purposes. Thus, comprehensive analysis of variable makers, glial and neuronal proteins, will be provide more precise and sensitivity clinical utility. Our study does not shown a significant correlation between serum brain specific protein level and the other demographic characteristics except hospital admission GCS score and parenchymal injury on radiologic examination. In previous study of correlation of S100B or NSE with GCS score, positive correlation was found 19). Some authors 32,33), reported there was no significant correlation between serum brain specific protein level and GCS score. The absence of correlation may be due to the fact that consciousness is often iatrogenically lowered when patients are intubated at the trauma scene with the use of anesthetics, sedative drugs, and neuromuscular blockade agents. One of the clinical implications of the current study may be that in patients in whom the GCS score cannot be reliably obtained in emergency department, blood specific protein levels in blood may be a good indicators for the severity of the brain damage. In parenchymal injury, direct neuronal and/or glial injury like contusion, may contribute to increase serum biomarker than focal mass lesion, pressure effect such as subdural or epidural hemorrhage. The pattern of brain injury is associated to difference elevation of markers. According to this result, better outcome may can be expected in mass effect without high level of markers than with high elevation. In result of the correlations, there was no significant correlation between glial and neuronal cell body marker. The brain consists of many elements such as neuronal cell body, axon, Schwann cell, glial cells, and CSF. According to the mechanism and severity of injury, various damage patterns are presented. Glial makers (S100B, GFAP) of each other belong to similar category but UCH-L1 is different. The glial cells damage is not certainly matched to neuronal cell damage (i.e., focal mass lesion versus diffuse injury). Different patterns of biomarker release imply that different patterns of structural damage involve different pathophysiological mechanisms and may require different therapeutic approaches. Because the S100B, GFAP, and UCH-L1 could not shows significant correlation to each other, multi-marker combined analysis reflecting different pathophysiology of each other is needed to predict for severity and clinical outcome.
Although these data showed encouraging result for prediction of severity of brain damage and clinical outcome, we recognize that there are limitations to this study. First, this study were performed in limited number of patients. Second, this study did not establish control group, and only compared with severe group and mild group. Thus, there are stratification errors, and the applicability of this result to patients is not definitive. Further study with larger cases and adequate control groups is required to assess the clinical application of these results.
CONCLUSION
The serum level of glial and neuronal biomarkers (S100B, GFAP, UCH-L1) at the time of admission were significantly correlated with clinical outcome after TBI and have reasonable sensitivity and specificity for determinating of severity and predicting important clinical outcomes. The identification of these markers would have clinically significant implications, including the diagnosis, prognosis and optimal treatment. The brain consists of many elements, not simple components. According to the mechanism and severity of injury, various damage patterns and different pathophysiology are presented. Hence, in clinical application of biomarkers, serum based combined multi-marker analysis reflecting glial and neuronal cell damage should be considered for understanding more precise pathophysiological state of brain and different therapeutic approaches.
Acknowledgements
This work was supported (in part) by Konyang University Myunggok Research Fund of 2013.
References
1. Alpert JS, Thygesen K, Antman E, Bassand JP : Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000, 36 : 959-969,   2. Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G : High serum S100B levels for trauma patients without head injuries. Neurosurgery 2001, 48 : 1255-1258, discussion 1258-1260   3. Baker SP, O'Neill B, Haddon W Jr, Long WB : The injury severity score : a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 1974, 14 : 187-196,   4. Bell MJ, Kochanek PM, Heyes MP, Wisniewski SR, Sinz EH, Clark RS, et al : Quinolinic acid in the cerebrospinal fluid of children after traumatic brain injury. Crit Care Med 1999, 27 : 493-497,   5. Berger RP, Beers SR, Richichi R, Wiesman D, Adelson PD : Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J Neurotrauma 2007, 24 : 1793-1801,   6. Berger RP, Pierce MC, Wisniewski SR, Adelson PD, Clark RS, Ruppel RA, et al : Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics 2002, 109 : E31,   7. Dimopoulou I, Korfias S, Dafni U, Anthi A, Psachoulia C, Jullien G, et al : Protein S-100b serum levels in trauma-induced brain death. Neurology 2003, 60 : 947-951,   8. Eccles MP, Foy R, Sales A, Wensing M, Mittman B : Implementation Science six years on--our evolving scope and common reasons for rejection without review. Implement Sci 2012, 7 : 71,     9. Hamm CW, Katus HA : New biochemical markers for myocardial cell injury. Curr Opin Cardiol 1995, 10 : 355-360,   10. Hammond FM, Grattan KD, Sasser H, Corrigan JD, Bushnik T, Zafonte RD : Long-term recovery course after traumatic brain injury : a comparison of the functional independence measure and disability rating scale. J Head Trauma Rehabil 2001, 16 : 318-329,   11. Hans P, Born JD, Albert A : "Extrapolated" creatine kinase-BB isoenzyme activity in assessment of initial brain damage after severe head injury. J Neurosurg 1987, 66 : 714-717,   12. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA : Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med 2008, 358 : 453-463,   13. Jackson P, Thompson RJ : The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J Neurol Sci 1981, 49 : 429-438,   14. Kleiman NS, Lakkis N, Cannon CP, Murphy SA, DiBattiste PM, Demopoulos LA, et al : Prospective analysis of creatine kinase muscle-brain fraction and comparison with troponin T to predict cardiac risk and benefit of an invasive strategy in patients with non-ST-elevation acute coronary syndromes. J Am Coll Cardiol 2002, 40 : 1044-1050,   15. Kruse A, Cesarini KG, Bach FW, Persson L : Increases of neuron-specific enolase, S-100 protein, creatine kinase and creatine kinase BB isoenzyme in CSF following intraventricular catheter implantation. Acta Neurochir (Wien) 1991, 110 : 106-109,   16. Middeldorp J, Hol EM : GFAP in health and disease. Prog Neurobiol 2011, 93 : 421-443,   17. Mishra A, Srivastava C, Singh SK, Chandra A, Ojha BK : Choroid plexus carcinoma : case report and review of literature. J Pediatr Neurosci 2012, 7 : 71-73,    18. Missler U, Wiesmann M, Friedrich C, Kaps M : S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke 1997, 28 : 1956-1960,   19. Missler U, Wiesmann M, Wittmann G, Magerkurth O, Hagenström H : Measurement of glial fibrillary acidic protein in human blood : analytical method and preliminary clinical results. Clin Chem 1999, 45 : 138-141,   20. Mondello S, Linnet A, Buki A, Robicsek S, Gabrielli A, Tepas J, et al : Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery 2012, 70 : 666-675,    21. Morganti-Kossmann MC, Hans VH, Lenzlinger PM, Dubs R, Ludwig E, Trentz O, et al : TGF-beta is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood-brain barrier function. J Neurotrauma 1999, 16 : 617-628,   22. Papa L, Lewis LM, Silvestri S, Falk JL, Giordano P, Brophy GM, et al : Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J Trauma Acute Care Surg 2012, 72 : 1335-1344,    23. Park JE, Kim SH, Yoon SH, Cho KG, Kim SH : Risk Factors Predicting Unfavorable Neurological Outcome during the Early Period after Traumatic Brain Injury. J Korean Neurosurg Soc 2009, 45 : 90-95,    24. Pelinka LE, Kroepfl A, Leixnering M, Buchinger W, Raabe A, Redl H : GFAP versus S100B in serum after traumatic brain injury : relationship to brain damage and outcome. J Neurotrauma 2004, 21 : 1553-1561,   25. Pickett W, Ardern C, Brison RJ : A population-based study of potential brain injuries requiring emergency care. CMAJ 2001, 165 : 288-292,   26. Raabe A, Grolms C, Sorge O, Zimmermann M, Seifert V : Serum S-100B protein in severe head injury. Neurosurgery 1999, 45 : 477-483,   27. Ross SA, Cunningham RT, Johnston CF, Rowlands BJ : Neuron-specific enolase as an aid to outcome prediction in head injury. Br J Neurosurg 1996, 10 : 471-476,   28. Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT, et al : Classification of traumatic brain injury for targeted therapies. J Neurotrauma 2008, 25 : 719-738,    29. Schäfer BW, Heizmann CW : The S100 family of EF-hand calcium-binding proteins : functions and pathology. Trends Biochem Sci 1996, 21 : 134-140,   30. Siman R, Toraskar N, Dang A, McNeil E, McGarvey M, Plaum J, et al : A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. J Neurotrauma 2009, 26 : 1867-1877,    31. Usui A, Kato K, Abe T, Murase M, Tanaka M, Takeuchi E : S-100ao protein in blood and urine during open-heart surgery. Clin Chem 1989, 35 : 1942-1944,   32. van Geel WJ, de Reus HP, Nijzing H, Verbeek MM, Vos PE, Lamers KJ : Measurement of glial fibrillary acidic protein in blood : an analytical method. Clin Chim Acta 2002, 326 : 151-154,   33. Yamazaki Y, Yada K, Morii S, Kitahara T, Ohwada T : Diagnostic significance of serum neuron-specific enolase and myelin basic protein assay in patients with acute head injury. Surg Neurol 1995, 43 : 267-270, discussion 270-271   34. Yoon KH, Han KS, Sung MM : Fabrication of a new type of organic-inorganic hybrid superlattice films combined with titanium oxide and polydiacetylene. Nanoscale Res Lett 2012, 7 : 71,    35. Zimmer DB, Cornwall EH, Landar A, Song W : The S100 protein family : history, function, and expression. Brain Res Bull 1995, 37 : 417-429,   36. Zoltewicz JS, Scharf D, Yang B, Chawla A, Newsom KJ, Fang L : Characterization of antibodies that detect human GFAP after traumatic brain injury. Biomark Insights 2012, 7 : 71-79,   
Fig. 1
Distribution of GCS score of S100B (A), GFAP (B), and UCH-L1 (C). GCS : Glasgow Coma Scale, S100B : S-100 beta, GFAP : glial fibrillary acidic protein, UCH-L1 : ubiquitin carboxy-terminal hydrolase-L1.

Fig. 2
Mean serum concentrations of biomarkers in each groups (severe/mild injured). S100B : S-100 beta, GFAP : glial fibrillary acidic protein, UCH-L1 : ubiquitin C-terminal hydrolase-L1.
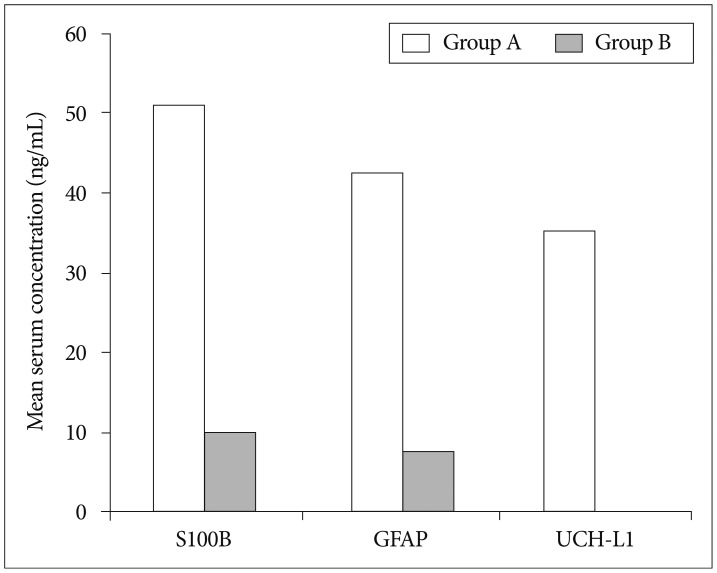
Fig. 3
Mean serum concentration of biomarkers in each groups (death/survivor). S100B : S-100 beta, GFAP : glial fibrillary acidic protein, UCH-L1 : ubiquitin C-terminal hydrolase-L1.

Fig. 4
Scatter plot of correlation between S100B and GFAP (A), UCH-L1 and S100B (B), and UCH-L1 and GFAP (C). S100B : S-100 beta, GFAP : glial fibrillary acidic protein, UCH-L1 : ubiquitin C-terminal hydrolase-L1.
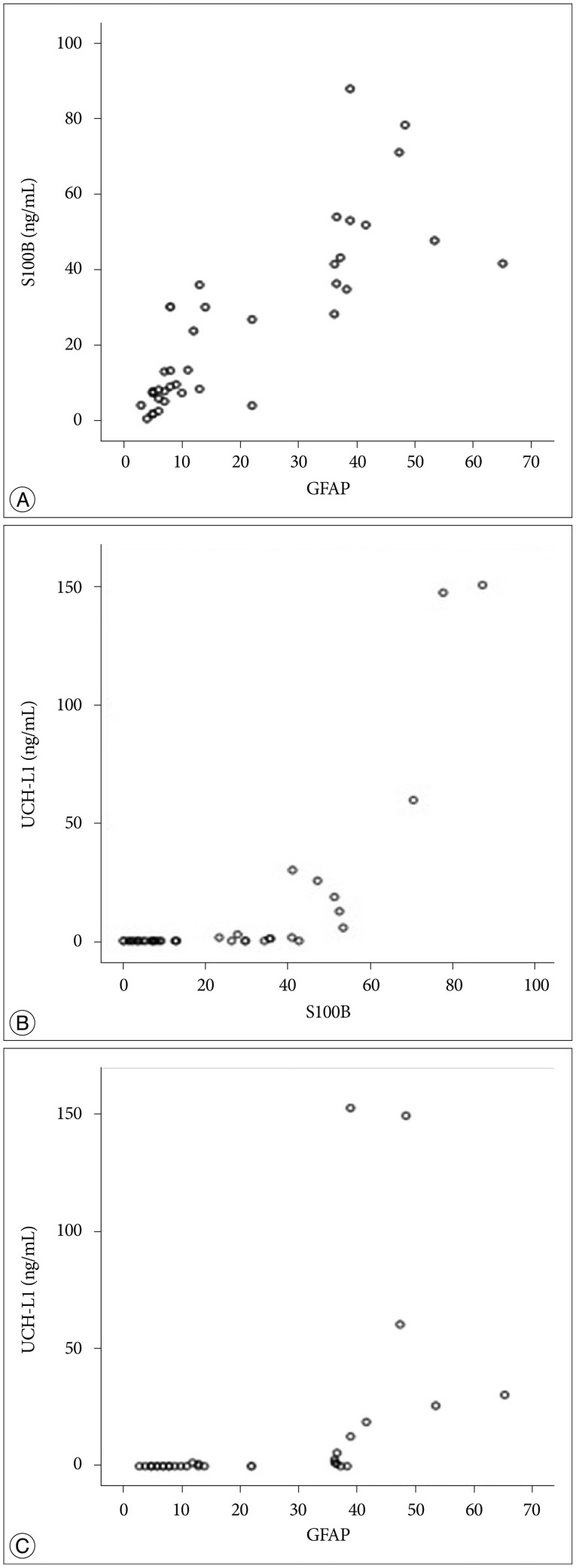
Fig. 5
Receiver operating characteristic curves of the biomarkers. S100B : S-100 beta, GFAP : glial fibrillary acidic protein, UCH-L1 : ubiquitin C-terminal hydrolase-L1.
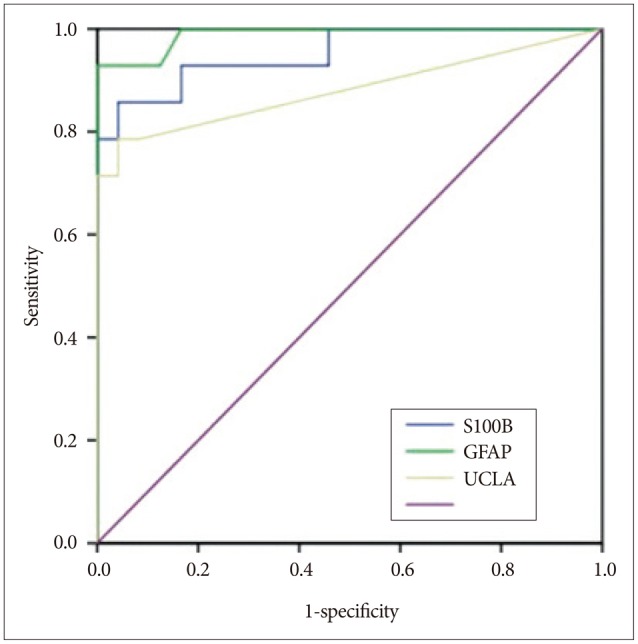
Table 1
Demographic characteristics and neurologic state on admission
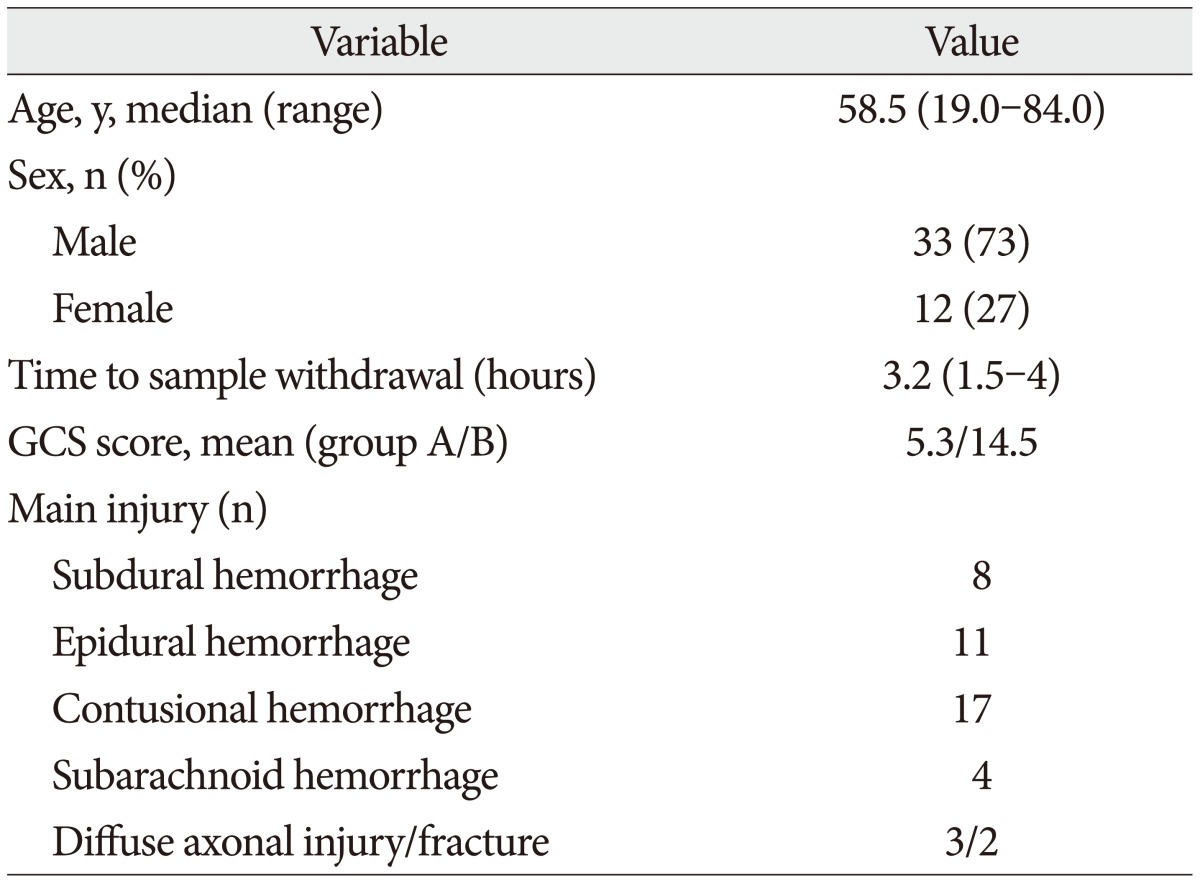
Table 2
Clinical outcomes after traumatic brain injury
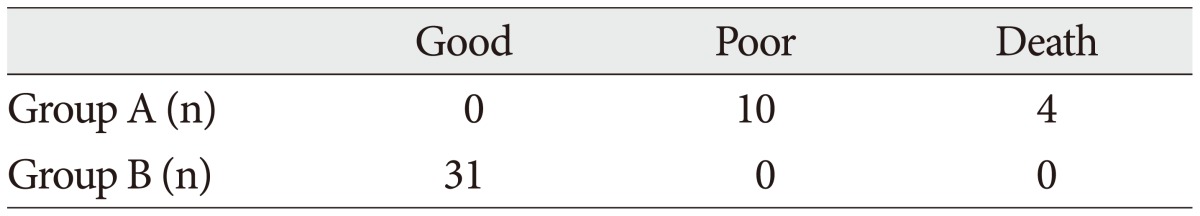
Table 3
Mean serum concentrations of biomarkers in each groups (severe/mild injured)
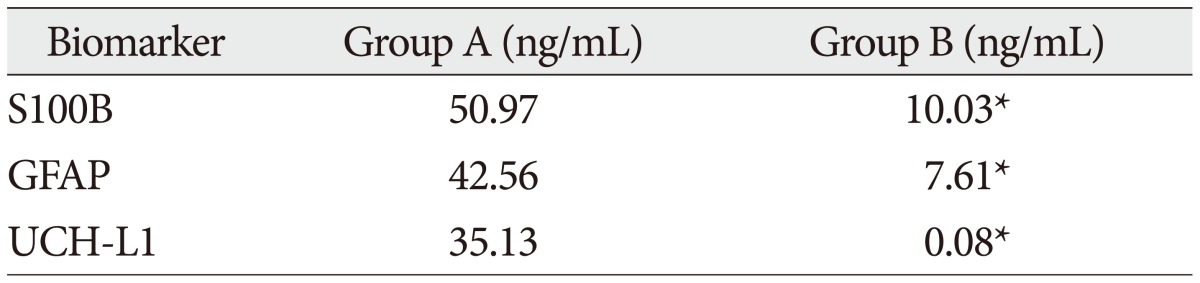
Table 4
Mean serum concentration of biomarkers in each groups (death/survivor)
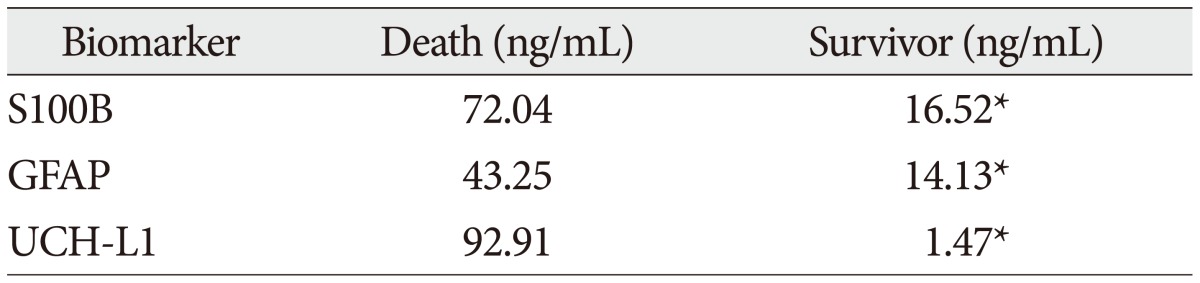
Table 5
Mean serum concentrations of biomarkers according to parenchymal injury on radiologic examination
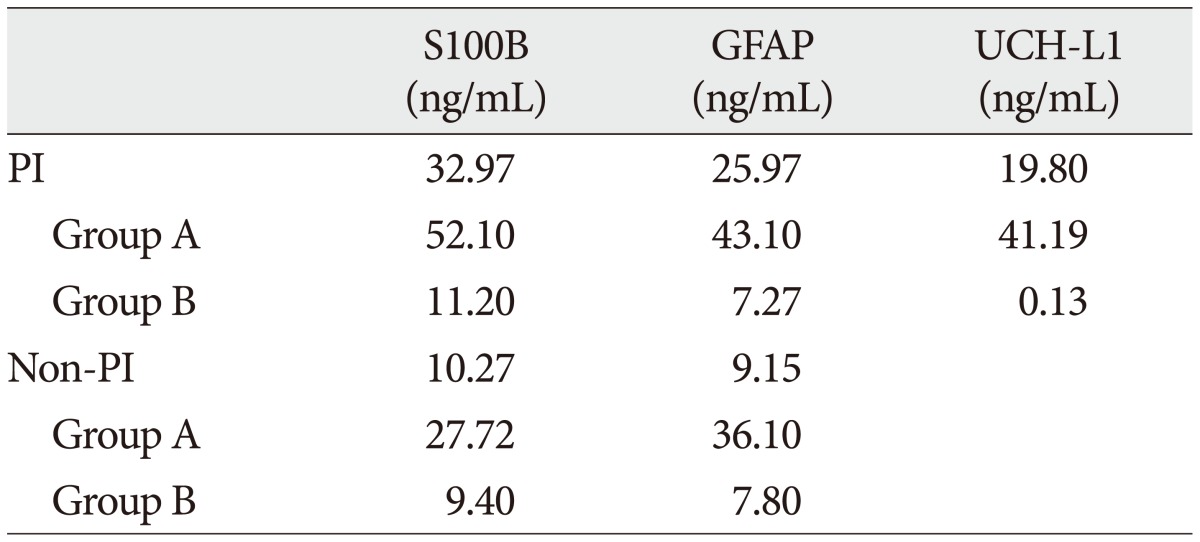
Table 6
Spearman correlation coefficient between serum concentrations and clinical variables
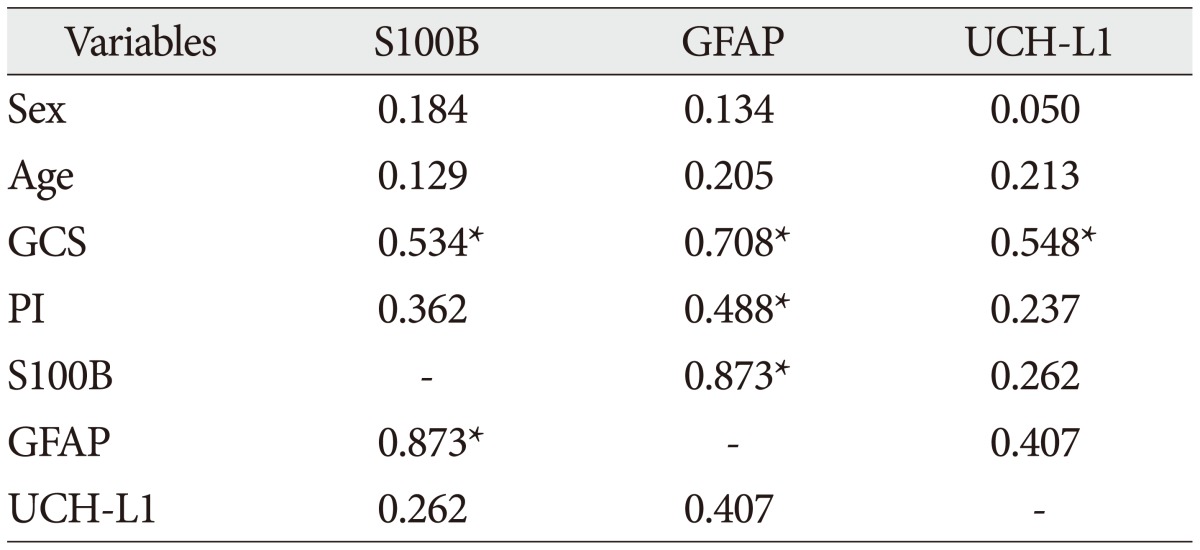
Table 7
Detection rate of poor outcomes by cutoff values
|
|



























