INTRODUCTION
Spontaneous intracranial hypotension (SIH) is a rare condition with a reported incidence of about 5 per 100000 individuals4). It is typically characterized by low cerebrospinal fluid (CSF) pressure, diffuse pachymeningeal enhancement, and postural orthostatic headache which gradually increases and can occur without a history of trauma or a dural manipulation procedure. SIH is caused by leakage of CSF from a tear in the dura at the level of the spine18). SIH is likely to present with a favorable clinical course and can resolve spontaneously or with a minimally invasive treatment (e.g., simple bed rest and epidural blood patch), but some complications such as chronic subdural hemorrhage (SDH)3,810,1117) and cerebral venous thrombosis6,17) are reported occasionally.
Chronic SDH is a common intracranial hemorrhage, but is often underestimated compared to other intracranial hemorrhages due to its benign course. Nevertheless, when associated with SIH and improperly treated, it may result in fatal consequences such as acute SDH development.
In this report, we present a unique case of chronic SDH associated with SIH which developed into extended pneumocephalus, and we also discuss pathophysiologic considerations.
CASE REPORT
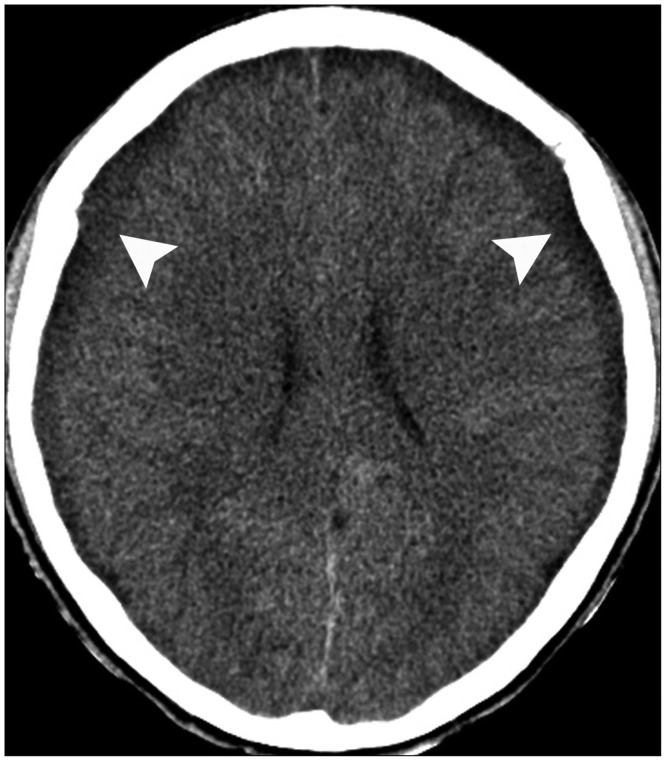
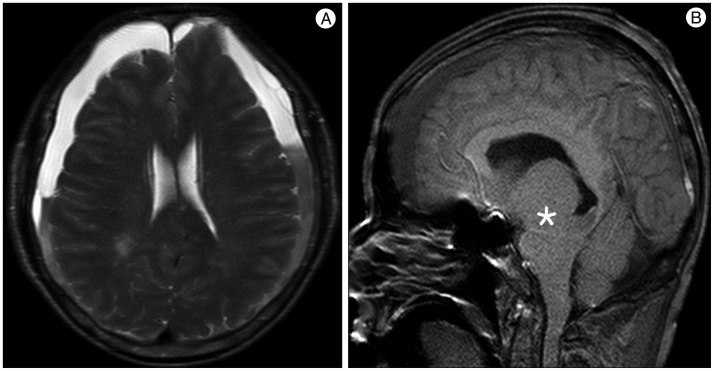
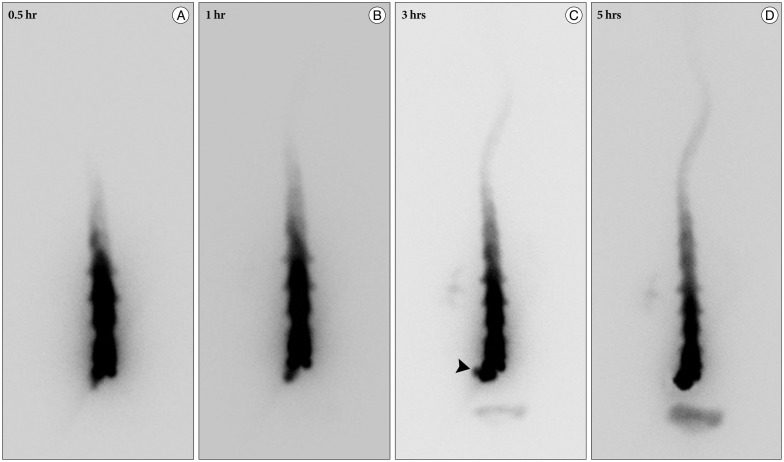
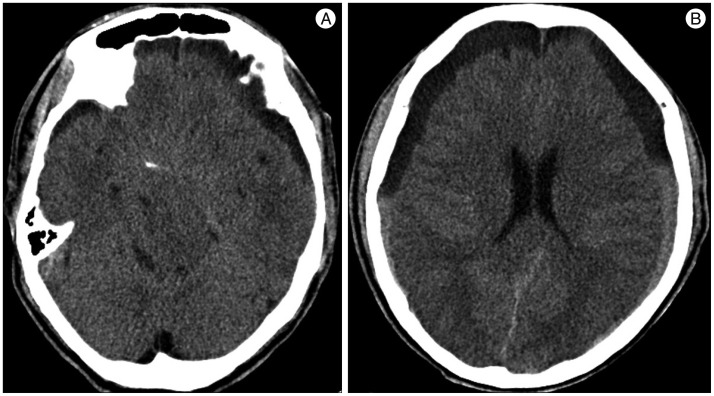
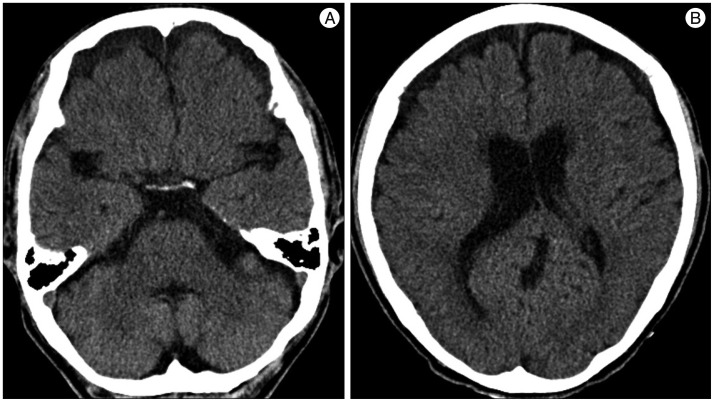
A 59-year-old male with a 1-month history of progressive postural headache and posterior neck pain visited the emergency room. He had no history of previous illness, minor trauma, or spine procedure. On neurological examination, he was alert and showed no abnormal neurological signs except for slight nuchal rigidity. Initial brain computed tomography (CT) showed lower subdural attenuating crescentic form fluid collection along the bilateral frontotemporoparietal area, which is typical of a SDH (Fig. 1). He was diagnosed with bilateral chronic SDH. In sequence, burr-hole trephination to evacuate the SDH was carried out. Surgical finding was similar to previous chronic SDH one, and after saline irrigation, efforts were made to fill the subdural space with saline to reduce pneumocephalus. Lastly, a drainage catheter was placed into the subdural space to drain the remaining SDH and air in the hematoma cavity. The procedure was uneventful. However, postoperative CT taken in 1 hour after surgery revealed an unusually extensive pneumocephalus that occupied both the frontal area, the subarachnoid space of both sylvian, and the basal cistern (Fig. 2). The patient awoke with no neurologic deficit, but he remained lethargic and complained of a severe headache. Brain magnetic resonance imaging (MRI) was performed 3 days later, and showed bilateral re-accumulation of subdural fluid collection and downward displacement of the midline structures with the brainstem, called "sagging" (Fig. 3). We suspected that CSF leakage was the underlying cause of intracranial hypotension and chronic SDH collection; radionuclide cisternography was performed with a lumbar intrathecal injection and whole cerebrospinal axis planar images were obtained 0.5, 1, 3, and 5 hours after injection. The 3-hour image revealed CSF leakages at the right distal sacral area (Fig. 4). Subsequently, an epidural blood patch (EBP) was performed via sacral hiatus using a 20-gauge needle and 10 mL of autologous blood was injected at the right S1-2 level. Despite strict bed rest for several days, the patient's headache persisted and did not improve. He became increasingly lethargic a week after the epidural blood patch and a follow-up brain CT scan revealed SDH enlargement with transtentorial herniation of the right-side parahippocampal gyrus (Fig. 5). We assumed that possible dural tear was too large for an epidural blood patch to seal the CSF leakage and that continuous leakage resulted in recurrence of the SDH and transtentorial herniation. To relieve the elevated intracranial pressure from the recurred SDH, we performed burr-hole trephination with hematoma evacuation. After surgery, the patient recovered with no neurological deficit, but postoperative brain CT scan showed similar degree of pneumocephalus as previous one, and remained lethargic for a couple of days. Afterwards, lumbar MRI was performed for exact anatomical evaluation of the leaking location, and revealed a large lobulating cystic lesion with a fistula in the sacral canal and right sacral foramen at the S2-upper S3 level, which were compatible with a large meningeal cyst (Fig. 6). A week after the 2nd subdural hematoma surgery, he was brought to the operating room for spine surgery to seal CSF leaking point on the meningeal cyst in S2-3. After drilling away of the right S2-3 posterior element, a large meningeal cyst was exposed and found to be connected with the right S3 root. There was a large dural defect on the inferior-lateral aspect of the meningeal cyst. The meningeal cyst was opened and packed with fascia and gelform, and finally sealed with fibrin glue, instead of ligating the cyst for the preservation of S3 root. The patient recovered without complications, and he was alert and well-oriented the day after surgery. Follow-up brain CT obtained 10 days after spinal surgery indicated that the subdural hematoma had resolved (Fig. 7) and the patient was doing well at the 6-month follow-up examination.
DISCUSSION
Chronic SDHs are relatively common in the elderly population and SDH pathophysiology has been considered a consequence of subdural space development due to age and cortical atrophy leading to the formation of a membrane around the hematoma12,1314). Although there is very little agreement about the optimal treatment strategy to manage chronic subdural hematomas, surgical treatment of symptomatic chronic SDH by a burr-hole procedure or craniotomy, with or without placement of a subdural drain, has been traditionally accepted20).
Usually, the cause of chronic SDH is attributed to minor head trauma in addition to some risk factors such as coagulopathy and brain atrophy1,15). However, in recent years, SIH is often reported to be associated with chronic SDH development. It is important to differentiate chronic SDH patients with underlying SIH from those without SIH because of the risk of recurrence despite repeated drainage10).
The SIH, first described by Schaltenbrand in 193821), is now believed to be caused by CSF leakage through small dural defects of the spine within the spinal epidural space, resulting in low CSF volume22). SIH is usually characterized by spontaneous postural headaches with neck stiffness, nausea, vomiting, tinnitus, and vertigo and SIH is defined by a CSF opening pressure lower than 60 mm H2O2,516,19). To understand SDH formation resulting from underlying SIH, Nardone et al.17) gathered previous reports and summarized that downward displacement of the brain due to low CSF pressure may produce tears in bridging veins of the dural border cell layer, causing these veins to rupture. Conversely, as subdural CSF collections gradually enlarge the subdural space, the bridging veins may stretch, and, in some cases, rupture.
The incidence of chronic SDH among SIH patients is reported to range between 20 and 40%10,1123). However, there is a lack of established or accepted management guidelines for chronic SDH patients with concomitant SIH. Some reports expressed that measures to stop CSF leakage, specifically epidural blood patches, are required to precede surgical drainage of chronic SDH3,9). In contrast one report indicated that SIH resolved spontaneously after surgical drainage of chronic SDH8).
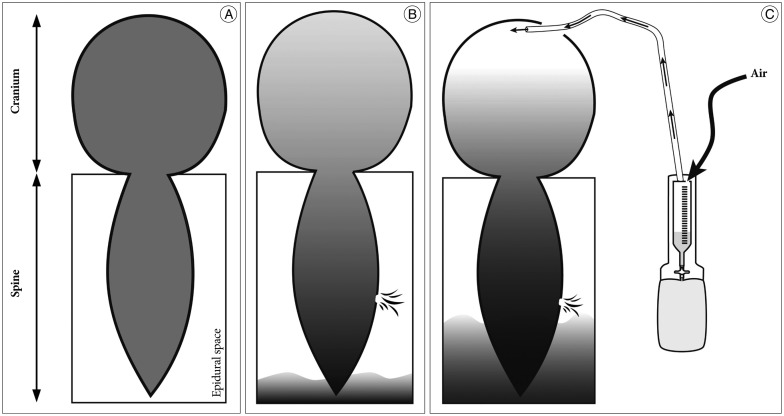
Surgical drainage of SDH was initially attempted to treat chronic SDH with SIH in our patient to relieve increased intracranial pressure and a drainage catheter was placed to evacuate the remaining SDH and entrapped air. However, unfortunately, air entered the cranial cavity via the drainage catheter which caused profound pneumocephalus. This phenomenon could be explained by the "inverted-soda-bottle effect" proposed by Horowitz7). Horowitz proposed that negative intracranial pressure results from excessive loss of CSF through an iatrogenic lumbar drain or settling into the distendable spinal subarachnoid space or simply drainage via normal pathway physiologic activities, such as inspiration or the Valsalva maneuver. However, when there are fistulous connections between the intracranial and outer spaces, air can enter the intracranial space in response to a negative pressure gradient. With a leakage in the spinal dura, which is an underlying cause of SIH, the fistulous connection was provided by the burr-hole drainage and the additional drainage catheter insertion. Finally, air might enter the intracranial space through the burr hole and the drainage catheter due to negative intracranial pressure, which was previously produced by the CSF leak caused by the spinal dura defect. Schematic drawings of these findings are presented in Fig. 8.
Although an EBP before surgical drainage of SDH could prevent such extensive pneumocephalus, SDH could recur soon after the procedure. Because the dural defect was too large to be sealed or compressed by a mere EBP, SIH was difficult to treat. In addition, there could be a chance to aggravate the CSF leakage in this patient during EBP via sacral hiatus, where the meningeal cyst located in nearby.
Although SIH is not easily determined to be the underlying cause of symptomatic chronic SDH, it should be in the differential diagnosis in identifying the cause of chronic SDH. When profound pneumocephalus is developed after burr-hole trephination for draining chronic SDH and SIH is suggestive, measures to localize the site of the leak and stop CSF leakage by EBP or repairing the dural defect should be performed. Nevertheless, in particular cases, performing emergency surgery to drain chronic SDH could be necessary in patients with impending brain herniation. This case report draws attention to the fact that a large spinal dural defect, the cause of SIH, should have been repaired prior to draining chronic SDH.