Destructive Radiologic Development of Intravascular Papillary Endothelial Hyperplasia on Skull Bone
Article information
Abstract
Intravascular papillary endothelial hyperplasia (IPEH) is a rare vascular benign lesion that rarely involves the central nervous system with or without skull invasion. We report a rare case of IPEH on the skull bone, which displayed destructive radiologic development associated with hemorrhage. A 14-year-old male presented with an incidentally detected a small enhancing, left frontal osteolytic lesion. Previously, he underwent operation and received adjuvant chemoradiation therapy for cerebellar medulloblastoma. Follow-up magnetic resonance imaging revealed a left frontal bone lesion, which expanded to an approximately 2 cm-sized well-circumscribed osteolytic lesion associated with hemorrhage for 20 months. Frontal craniectomy and cranioplasty were performed. Destructive change was detected on the inner table and diploic space of the skull. The mass had a cystic feature with hemorrhagic content without dural attachment. Pathologic examination showed the capsule consisted of parallel collagen lamellae representing a vascular wall, vascular lumen, which was pathognomonic for IPEH. Immunohistochemical staining revealed that the capsule was positive for CD34 and factor VIII, which favor the final diagnosis of IPEH. This was the first case of intracalvarial IPEH.
INTRODUCTION
Intravascular papillary endothelial hyperplasia (IPEH) is a rare vascular benign lesion. It is generally considered to be a benign proliferation of endothelial cells with secondary thrombosis and fibrin deposition or an excessive reaction to the normal reorganization process in a thrombus2,18). The lesion contains papillary structures composed of a single layer of swollen endothelial cells around a core of fibrous connective tissue and may be mistaken for angiosarcoma based on histopathological findings12). IPEH most commonly occurs in the skin and subcutaneous soft tissues, where it exhibits a benign clinical course. But, it can also occur in the nasal cavity, pharynx, larynx, internal auditory canal, labyrinthine, heart valve, breast, digestive tract, liver, kidney, cervix, uterus, female urethra, and pelvic veins12,17). IPEH associated with the central nervous system, with or without skull invasion, is rare9). We describe such a case of IPEH located on skull that revealed destructive change.
CASE REPORT
A mass on the left frontal bone was incidentally detected in a 14-year-old male. The patient had underwent an operation for cerebellar medulloblastoma 4 years ago. After the operation, adjuvant radiotherapy consisted of craniospinal irradiation and chemotherapy had been given according to the M-051 protocol of the Korean Society of Pediatric Neuro-oncology. Two years post-operatively, dilated or prominent vascular structures were detected around the left frontal bone marrow on gadolinium (Gd)-enhanced axial T1-weighted magnetic resonance imaging (MRI) (Fig. 1A). Six months later, a follow-up T2-weighted MRI revealed a well-defined hyperintense nodular lesion in the same area with homogeneous enhancement (Fig. 1B). We presumed that the lesion was a benign bone marrow lesion, such as hemangioma, rather than metastasis. However, a routine follow-up axial Gd-enhanced MRI 7 months later demonstrated that the lesion was a well-circumscribed growing cystic mass with thin peripheral enhancement. Prompted by this latest finding, we began to consider the possibility of a metastatic lesion. Further growth of the rim enhanced cystic mass was evident upon examination 7 months later (Fig. 1C). Gradient echo imaging revealed newly developed internal hemorrhage in the cystic mass (Fig. 1D). Computed tomography showed a well-circumscribed, expansile osteolytic mass located on the inner table and diploic space of the frontal bone (Fig. 1E). During the 20 months of follow-up, the lesion had grown slowly to a 2 cm-sized cystic osteolytic lesion, which was suggestive of a metastatic bone tumor.
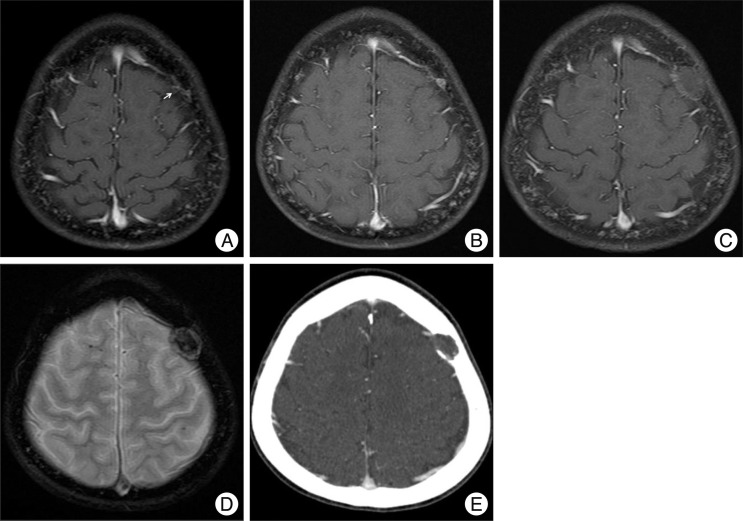
Radiologic findings with time. A : Two years post-operatively, dilated or prominent vascular structures (arrow) were detected around the left frontal bone marrow. B : Six months later, follow-up MRI reveals a well defined enhanced nodular lesion. C : Another 7 months later, growth of the rim enhanced cystic mass was evident. D : On a gradient echo image, newly developed internal hemorrhage is observed in the cystic mass. E : Computed tomography reveals a well-circumscribed, expansile osteolytic mass located on the inner table and diploic space of the frontal bone.
Frontal craniectomy including complete excision of the mass with safety margin was done and cranioplasty with bone cement was applied. Intra-operatively, the skull showed destructive changes of the inner table and diploic space, and the hemorrhagic content under the thin lining capsule (Fig. 2). Dural invasion was not evident. Pathologic findings showed both the capsule consisted of parallel lamellae of collagen and a vascular wall, vascular lumen, and papillary projection into the lumen associated with hemorrhage and calcification (Fig. 3A). There was no evidence of cytologic atypia, abnormal mitosis, necrosis, or any solid spindle-cell areas. The mass was immunopositive for CD34 and factor VIII (Fig. 3B). The final diagnosis was IPEH. No post-operative complications occurred and no recurrence was evident at the 1-year follow-up.
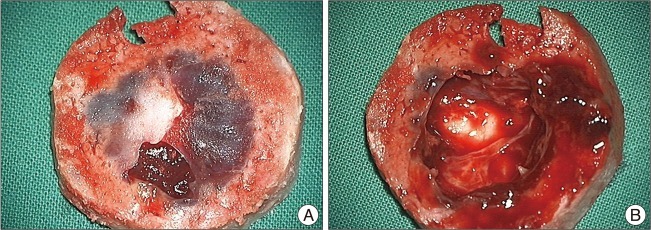
Operative findings. A : The of the skull shows destructive change of the inner table with diploic space. B : The mass shows a necrotic and hemorrhagic morphology with lining of the thin capsule.
DISCUSSION
IPEH was described in 1923 for the first time, and is known by many eponyms in the literature, including Masson's vegetant intravascular hemangioendothelioma14), intravascular angioma-tosis18), Masson's pseudoangiosarcoma12), intracranial Masson's hemangioma4), and intravascular papillary endothelial hyperplasia22). It generally appears as a subcutaneous or intramuscular nodule on the finger, in the head and neck region, or in thrombosed vessels, principally the hemorrhoidal veins18,22). Intracranial occurrence is very rare, with only a few cases being reported to involve the on central nervous system with or without skull invasion9).
IPEH is generally considered to be a specific form of thrombus organization, rather than a primary intravascular tumor2,18). Three forms of IPEH have been described10). The primary type is an unusual reactive response of normal endothelial cells to thrombus formation, almost always in low flow venous channels. The lesion impairs the venous drainage system, which causes the congestion and hemorrhage24). The secondary type is associated with pre-existing cavernous hemangioma and hemorrhoidal veins complicated by thrombosis and the extravascular form develops in extravascular organized hematomas17). Intracranial occurrence of IPEH is very rare, with only 18 cases reported9). It presents as a slowly growing lesion that develops by thrombosis and endothelial proliferation. However, a rapid increase in size could require hemorrhage9).
Radiologically, the 18 previously described cases of intracranial IPEHs were located on the cerebral hemisphere in nine patients, cavernous sinus and sellar in three patients, cerebellum in two patients, brain stem in one patient, posterior inferior cerebellar artery in one patient, within the internal auditory canal in one patient, and in the superior orbital fissure in one patient9). Contrast enhancement has been documented in 61% of cases1,3-7,11,13,16,20) and associated hemorrhage has been described in 44.4% of cases5-9,19-21). Underlying vascular malformations such as cavernous angioma, venous angioma, and arteriovenous malformation have been described in 22.2% of cases8,11,16,21). Three cavernous sinus and sellar and two temporal IPEHs (27.7%) involved the destructive invasion of adjacent bone1,11,20). One case occurred in the skull of the superior orbital fissure16). This case was the first intracalvarial IPEH.
The present location of the IPEH on the skull is rare. During 20 months of observation, the destructive development of the lesion became apparent. Prominent vascular structures were detected around frontal bone marrow at first. A follow-up MRI revealed a homogeneously enhanced mass, which would be consistent with thrombosis and endothelial proliferation. This lesion became a well-circumscribed cystic mass with thin peripheral enhancement, consistent with repeated hemorrhaging, which may have driven the development of the mass. During the period of examination, the lesion grew slowly to form a well-circumscribed, expansile osteolyic mass located on the inner table and diploic space of the frontal bone. This benign lesion could have caused the lysis of the skull bone.
The diagnosis of IPEH can be made based on the microscopic examination23). Histologically, it is characterized by papillary proliferations and thrombotic material within the vascular lumen associated with a normal endothelial lining. It must be differentiated from other malignancies such as angiosarcoma and Kaposi's sarcoma18). Endothelial atypia, pleomorphism, necrosis, multiple layers of endothelium, and frequent mitoses are not supportive of a diagnosis of IPEH23). Immunohistochemistry data adds to the confidence of the diagnosis, since IPEH typically is positive for factor VIII-related antigen and CD34.
Treatment of IPEH is complete surgical excision, which yields the best outcome5,7,21). Recurrence has been noted in cases of subtotal resection1,11,15,19). Because of the small number of reported cases, it is still difficult to draw conclusions as to the efficacy of adjuvant treatment. Various doses of radiation have been used; some have been effective1,11). Likewise, the clinical outcome of chemotherapy remains vague19).
CONCLUSION
We reported the first intracalvarial IPEH, which showed destructive radiologic development associated with hemorrhage.
