Surgical Treatment of Giant Serpentine Aneurysm of A2-A3 Segment Distal Anterior Cerebral Artery : Technical Case Report
Article information
Abstract
Objective
To report our surgical experience using in situ end-to-side bypass for giant serpentine distal anterior cerebral artery aneurysm, unsuitable for microsurgical clipping.
Methods
A 49-year-old woman presented with headache and intermittent loss of consciousness. The brain computed tomography scan revealed a partially calcified mass in the interhemispheric fissure. On cerebral angiography, that was giant (30×18 mm sized), serpentine aneurysm originating from the A2 to A3 segment of the distal anterior cerebral artery (DACA). The aneurysm was trapped with clips, and the right A3 segment to left A3 segment of DACA, end-to-side in situ bypass was performed. Surgical result was favorable, with no newly developed ischemic event in the acute recovery period. Postoperative angiography showed total occlusion of the aneurysm and good patency, with preserved distal flow.
Conclusion
Giant fusiform aneurysms of the DACA are extremely rare and can be particularly challenging to treat. End-to-side A3 : A3 bypass with aneurysm trapping could be a treatment modality for these locations.
INTRODUCTION
Intracranial aneurysm can be optimally treated by either microsurgical clipping or endovascular coiling. However, both treatment modalities may be impossible in certain situations, such as giant or fusiform thrombosed aneurysm, in which efferent or adjacent arterial branches may be involved. Under these situations, an alternative surgical strategy using revascularization may be required6,7). Although revascularization using harvested graft vessels has been used for high-flow replacement in proximal vessels, local techniques of revascularization without a graft (in situ) may be sometimes more useful for distally located aneurysm. We report our surgical experience using in situ bypass technique for the treatment of giant serpentine distal anterior cerebral artery (DACA) aneurysm.
CASE REPORT
A 49-year-old woman presented with loss of consciousness (LOC) and history of headache. On initial neurologic examination, she had no specific symptoms or previous seizure history. A non-contrast computed tomography scan showed a 3×1.8 cm sized, rim calcified, and round shaped mass lesion in the interhemispheric area, along the anterior falx above the head of the corpus callosum (Fig. 1A). Brain T2-weighted structural magnetic resonance image (MRI) scan revealed partially thrombosed, signal voided aneurysm, without parenchymal edema (Fig. 1B). Cerebral angiography demonstrated an irregular serpentine aneurysm, involving the entire A2 segment of DACA, measuring 4.5 cm in the anteroposterior dimension and 1.8 cm in the transverse dimension (the proximal portion of aneurysm, Fig. 1C, D, E).

Non-contrast computed tomography demonstrating a 3×1.8 cm sized, rim calcified, and bilobulated mass lesion in the above corpus callosum. B : Brain T2-weighted magnetic resonance image revealed partially thrombosed, signal voided aneurysm. C : Cerebral angiography showing a 4.5×1.8 cm sized, irregular serpentine aneurysm. D : 3-D angiography image of the aneurysm involving the entire A2 segment of the distal anterior cerebral artery. E : Entire scheme of distal anterior cerebral artery serpentine giant fusiform aneurysm.
Surgical technique
The patient was given aspirin (100 mg) for elective operation. The patient was placed in the supine position, with the head slightly tilted at 20° degrees in the 3 pin fixation. The right calf lesion was prepped for possible bypass, using a venous interposition graft from the superficial temporal artery to the distal end of the anterior cerebral artery. To secure the wider surgical field, we performed cerebrospinal fluid (CSF) drainage via lumbar puncture and midline bifrontal interhemispheric craniotomy with the neuronavigation system (Medtronics®). 4×4 cm sized bone flap was made, then dura mater was opened with small straight incision. The superior sagittal sinus was ligated, cut, and reflected up and downward bidirectionally (Fig. 2A). Under the operating microscope, we advanced with dissection of the interhemispheric fissure and indentified the distal end of the fusiform aneurysm (Fig. 2B). After further dissection, the left pericallosal artery was indentified just adjacent to the aneurysm. Thus, at this point, we considered that it might be possible to perform the in situ bypass between right A3 just distal aneurysm sac and left A3. To see the proxymal portion of aneurysm and parent artery, we further dissected the interhemspheric fissure toward anterior direction and were very cautious not to injury the both olfactory nerves (Fig. 2C). This complication can be avoided by making wide, precautious dissection to minimize posterior and lateral brain retraction. The aneurysm wall itself was hardly adhesive to both frontal cortex. Therefore, we did not fully dissect the aneurysm from the brain, in order to avoid postoperative parenchymal contusion.

Intraoperative findings. A : 4×4 cm sized bone flap is made and dural flap is reflected after cutting the superior sagittal sinus. B : Atherosclerotic distal end of aneurysm is seen at the top. C : Both olfactory nerves are intact and dissected well. D : End to side bypass. E : Atherosclerotic, calcified aneurysm with small opening for blood aspiration.
Blood flow was measured at approximately 20 cm/s, in both distal artery to the fusiform aneurysm and opposite left pericallosal artery. When we placed the temporary clip on the proximal parent artery of aneurysm, blood flow was not visible on the micro-Doppler recording. Thus, we finally decided to perform aneurysm trapping with a right end-to-left side in situ bypass, to preserve the flow to the right DACA.
Before anastomosis, the patient was placed into burst suppression with propofol, and the mean arterial pressure was raised 25% above the patient's baseline, in order to improve the collateral circulation. Intraoperatively, both somatosensory and motor evoked potentials were also used for monitoring. At the time of the anastomosis, 2000 IU of heparin was given intravenously.
Both arteries were dissected as movable as possible, and a rubber dam was passed under both vessels. After placing two temporary clips on the right side, the distal end of the fusiform aneurysm was cut with sharp microscissors. The atherosclerotic distal artery of the aneurysm was clearly trimmed with jeweler forceps, and marked with marking pen. Two temporary clips were then placed on the left side, and following heparin irrigation, a small arteriotomy was made equal to the opposite arterial opening, using a diamond knife (FD 115D Aesculap, PA, USA). A marking pen is usually used to color the borders of the arteriotomy for idenfication of the margin of the transparent thin vessels. After both toe and heel stitches were placed at the proximal and distal ends of the anastomosis with 10-0 nylon suture (Ethicon Co., NJ, USA), the posterior wall was sutured in a continuous fashion and the anterior wall was sutured in an interrupted pattern. On completion of the end-to-side bypass, robust blood flow in the right DACA was observed in the distal segment to the bypass site (Fig. 2D). Temporary clipping time was around 50 minutes. Permanent clips were applied proximal and distal to the aneurysm, without removal of the thrombosed aneurismal mass, because the aneurysm itself was severely stuck to the brain surface. Instead of leaving the thrombosed aneurysm, we aspirated the blood clot from the aneurysm using 14-gauzed needle, to identify total obliteration of the aneurysm and to reduce the mass effect postoperatively (Fig. 2E). After meticulous bleeding control, closure was done in the customary fashion.
Postoperative evaluations
The patient was neurologically intact immediately after surgery. Immediate postoperative CT scan revealed no radiological abnormaliy (Fig. 3A). Postoperative cerebral angiography on day 7 demonstrated no residual filling of the aneurysm and excellent flow through a patent bypass into both the DACA vascular territories (Fig. 3B, C). Follow up T2-weighted brain MRI at 3 months after surgery showed remarkably reduced mass size in the interhemispheric area, along the genu portion of corpus callosum (Fig. 3D).
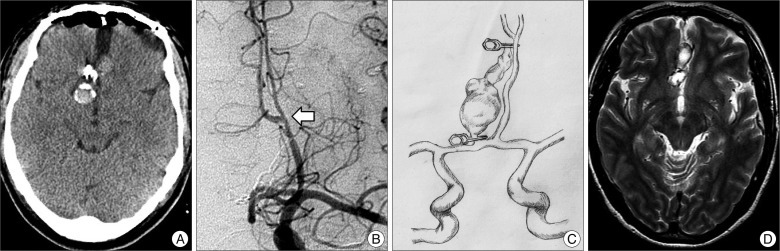
A : Postoperative computed tomography scan showing a placed clip and no hemorrhagic contusion. B : Follow-up angiography demonstrating good patency of bypass (arrow). C : Follow-up T2-weighted magnetic resonance image showing reduced aneurysm size. D : Follow-up T2-weighted magnetic resonance image showing reduced aneurysm size.
DISCUSSION
Since the first bypass reported in 1986 by Ikeda, including the microvascular side-to-side anastomosis technique3), the concepts and techniques for bypass have evolved over time and diverse surgical methods have been tried and evaluated for cerebral revascularization, in order to get the best surgical outcomes. The ideal surgical technique is to make an artificial conduit for blood flow between two adjacent vessels of similar caliber. This type of in situ bypass has been often applied for the treatment of complex brain aneurysms6,7).
The overall incidence of DACA aneurysms has been known to be from 2% to 9% of all intracranial aneurysms4,5); however, only 0.6% were fusiform, and is still unclear whether these were giant aneurysms. Dunn et al.1) were the first to demonstrate the technique as well as challenges of performing the A3 : A3 bypass to treat a large fusiform aneurysm located in the DACA. Furthermore, giant serpentine aneurysms in the DACA are very rare. Thus, this presented case is only the second reported end-to-side in situ A3 : A3 bypass with aneurysm trapping. Furthermore, to the best of our knowledge, giant serpentine aneurysm has not been reported previously in the literature in this DACA location. Herein, we described a successful surgical experience for the treatment of giant serpentine DACA aneurysm.
Considering surgery in the interhemisheric lesion, the operation field is quite deep and narrow that we often have difficulty with performing surgery in this location. Furthermore, DACA aneurysm is sometimes adhesive to the cingulated gyrus and often has wide or atherosclerotic neck, with difficulty to confirm the parent artery and surrounding structures5). Therefore, it is important to secure wider surgical field and brain relaxation, using CSF drainage and infusion of hyperosmolar fluid. Although this type of bypass surgery may be technically challenging because of aforementioned obstacles, meticulous dissection and enough surgical space can lead to successful completion of the anastomosis.
Dunn et al.1) reported one bypass for large (17 mm) fusiform A3 aneurysm, with resection of the aneurysm after end-to-side bypass. Aneurysmal resection was impossible in our case, because the aneurysm was 4.5 cm in length and too large to resect. The patient presented in this case was neurologically intact preoperatively. The goal of surgery was total obliteration of the aneurysm, with preservation of blood flow to the parent vessel territory and bypass revascularization in the end. Therefore, we did not remove the trapped aneurysm, to avoid injury of the adhesive tissue between the brain and the aneurysm wall. Fortunately, on the follow-up MRI scan (Fig. 3B), the mass size was decreased and the patient was symptom free postoperatively.
In a recent study, Gelfenbeyn et al.2) suggested that venous graft should be prepared, if necessary, to be used as interposition graft between the superficial temporal artery and the distal artery of the resected aneurysm. If there is a large, broad neck A2-A3 aneurysm, it might be difficult to perform the end-to-end anastomosis after aneurysm resection. As previously suggested, we also prepared the venous interposition graft in the patient's calf, but we did not use it, because this procedure should be tailored to the operative findings and availability of donor and recipient vessels.
CONCLUSION
Giant serpentine aneurysm in A2-A3 are very rare and can be difficult to treat with standard surgical methods, because of their atherosclerotic pathology and characteristically narrow surgical field. We describe an end-to-side in situ bypass technique combined with aneurysm trapping.