Supratentorial Intracerebral Schwannoma : Its Fate and Proper Management
Article information
Abstract
Intracerebral schwannomas are rare and there have been none reported in Korea. We present the case of a 25-year-old man with newly developed right-side weakness and recent seizure aggravation. His seizures started approximately 9 years prior to admission. At that time, a 1 cm diameter intra-axial enhancing mass at the left precentral gyrus was found on magnetic resonance image (MRI). After 9 years of observation and treatment with antiepileptic medication, an MRI taken due to symptom aggravation revealed peri-tumoral cyst formation with tumor enlargement. The tumor was surgically removed. Subsequently, right-side weakness diminished and there was good seizure control. Pathologic diagnosis was schwannoma. Schwannoma is a very rare tumor and there are no pathognomonic findings on radiologic images; thus, it is challenging to make a correct diagnosis. However, considering the natural course and excellent prognosis after surgical treatment of this kind of intra-axial mass with benign features, early surgery for diagnosis and proper treatment is highly recommended.
INTRODUCTION
Intracranial intraparenchymal schwannomas, which are unrelated to the cranial nerves, are very rare. Since Gibson et al.7) reported the first case of an intracerebral schwannoma located at the temporal lobe in 1966, about 70 cases have been reported in the English-language literature. Although some general characteristics of these tumors have been described, preoperative diagnosis is very challenging due to its rarity in the central nervous system and because of the absence of pathognomonic signs on radiologic images. Often, these challenges lead to delay in proper treatment of the patient.
To our knowledge, this is the first case of a supratentorial intracerebral schwannoma reported in Korea. The patient had an exceptionally long preoperative observational period (9 years) which allowed us to clarify the clinical and radiological course of this rare tumor. Based on our observations and a review of the relevant literature, we suggest early diagnosis and proper treatment of these rare tumors.
CASE REPORT
History and examination
A 25-year-old man presented with recent aggravation of focal seizure activities on the right arm was referred to our clinic. His first seizure attack took place 9 years ago which started with involuntary right arm tremors followed by a loss of consciousness and generalized tonic-clonic convulsions. When he first visited the neurology clinic at that time, a neurologic examination revealed no abnormalities. He had no relevant medical history, no stigmata of neurofibromatosis and no family history of this disorder. His magnetic resonance images (MRI) of the brain revealed a small mass measuring 1.5×1.0×1.1 cm at the left precentral gyrus. The mass was predominantly hypointense to gray matter on the T1 weighted images, heterogeneously hyperintense on the T2 weighted images and showed intense enhancement on gadolinium injection (Fig. 1). Peritumoral edema was observed, affecting mainly the left precentral gyrus. No cystic change was found. Initial radiologic differential diagnoses were a pleomorphic xanthoastrocytoma, ganglioglioma, and dysembryoplastic neuroepithelial tumor. Since he was free of symptoms between seizure attacks and well tolerated his condition after taking anti-epileptic medication, he refused to undergo surgical treatment. During the 9 years of follow-up, he had no trouble in carrying on with his daily life. He complained of only intermittent focal seizures presented as right arm tremors and transient weakness, and these seizure attacks mostly occurred under stress conditions like sleep deprivation and heavy alcohol consumption combined with poor drug compliance. A brain MRI which was taken two years after the initial image work-up, revealed no interval change. Because the patient's symptom was stable, no further imaging study was pursued.
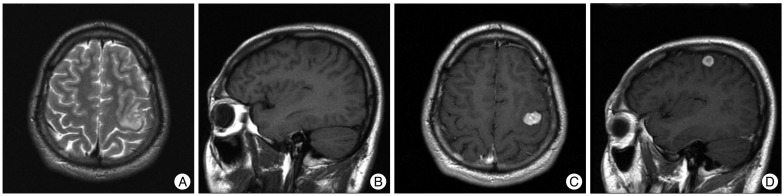
Brain MRI after the first seizure attack revealed a small mass lesion at the precentral gyrus with peritumoral edema. The mass is of heterogeneously high signal intensity on a T2-weighted axial image (A) and predominantly hypointense to gray matter on a T1-weighted sagittal image (B). It enhances homogeneously after gadolinium injection (C and D).
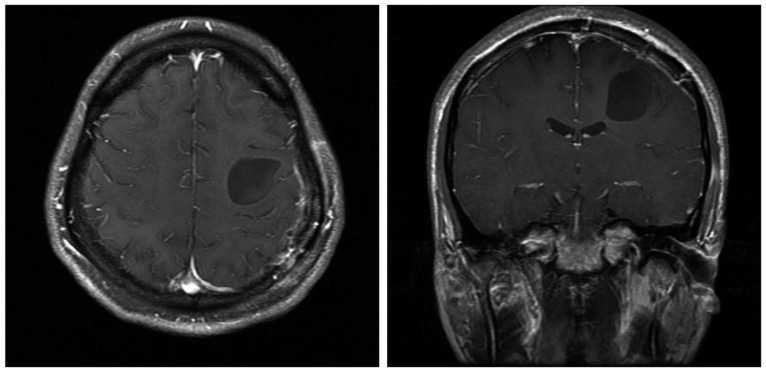
Nine years later, he complained of sudden prolonged seizure duration with right arm weakness, which became progressively worse over the past month prior to his visit. A neurological examination revealed a grade IV weakness of the right elbow and hand. Immediate brain computed tomography and MRI were taken. The size of the enhancing portion had increased to 2.3×1.9×1.6 cm, and a large peritumoral cyst measuring about 5.5 cm in diameter had newly appeared (Fig. 2). No intratumoral calcification and cystic wall enhancement were observed.
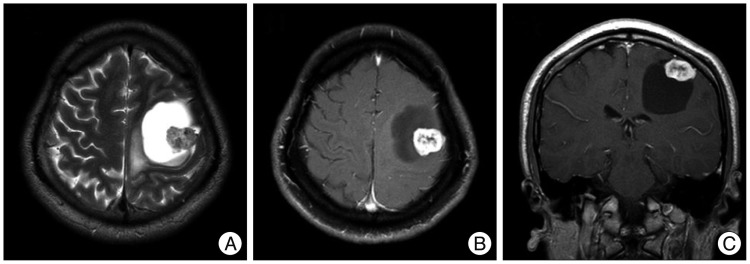
9-year follow-up brain MRI revealed a slightly increased enhancing mass with a large, newly appeared peritumoral cyst. The cyst is hyperintense on a T2-weighted axial image (A), and hypointense on a T1-weighted axial and coronal images (B and C). Cystic fluid shows a similar signal as the cerebrospinal fiuid.
Operation
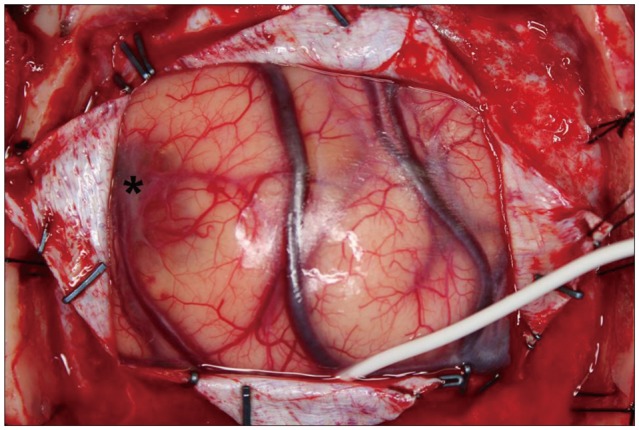
Since the mass was located at the precentral gyrus, awake craniotomy was performed to minimize postoperative motor and sensory deficit. A rectangular fronto-parietal craniotomy was done to expose the enhancing mass with the help of a neuronavigation system. Sonography was used to identify the mass located just beneath the cortical surface which appeared to have a bluish discoloration (Fig. 3). By direct cortical stimulation with a nerve stimulator, we confirmed that the motor cortex of the right wrist and hand were located just on the posteromedial side of the mass. Dissection started from the anterior part of the mass. It was well circumscribed from adjacent brain tissue and could be removed in an en bloc fashion. During removal, slight yellowish but cerebrospinal fluid like clear fluid gushed out from the cyst. The patient did not present any worsening of neurologic deficit during the surgery.
Postoperative course
The patient's postoperative course was uneventful and his right forearm and hand weakness improved. One month after the operation, he had a postoperative MRI which revealed no residual enhancing mass, but the cyst remained although its size was somewhat decreased (Fig. 4). At one year and six months of follow-up, he has remained seizure free since the operation and his right hand and arm weakness has recovered to near normal status, with no difficulty in his daily living.
Pathologic examination
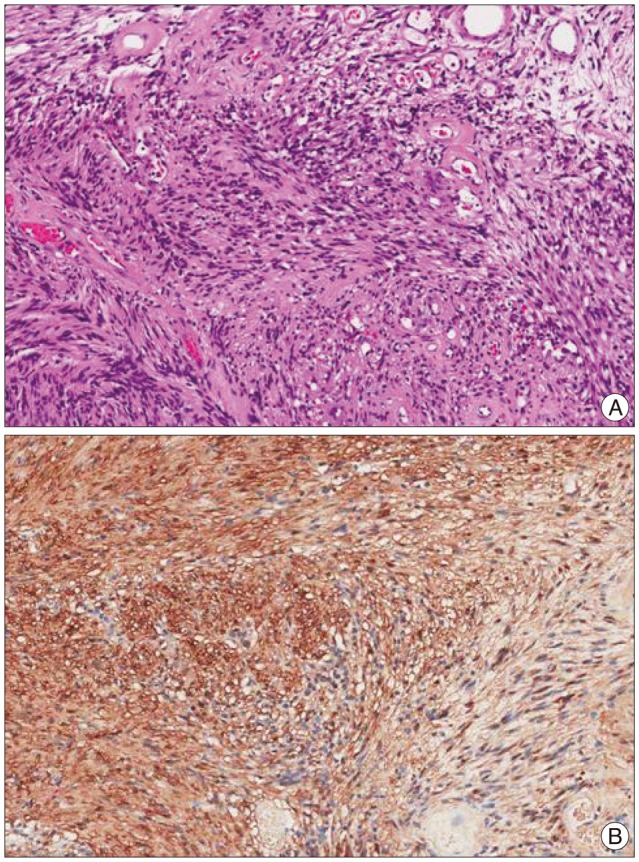
The specimen consisted of several fragments of soft tissues, measuring 2.0×1.0 cm in the largest dimension. Grossly, the cut surfaces showed a gray yellow myxoid appearance. Under light microscopy, the tumor was composed of alternating Antoni A and B areas, i.e., cellular and less cellular areas (Fig. 5). There was neither hemorrhage or necrosis or cystic change. The tumor had fascicular arranged spindle cells with elongated nuclei without nuclear pleomorphism. There was few mitoses (1/10 HPF) and the MIB-1 (Ki67) labeling index was low (1.03%). The tumor cells were robustly positive for S100 protein. Electron microscopic examination was consistent with Schwannoma, which showed elongated cells surrounded by a continuous external lamina and scattered Luse bodies (long space collagen).
DISCUSSION
Although intracranial intraparenchymal schwannomas are rare, we have developed knowledge of these tumors thanks to the case reports that have accumulated since 1966. Excluding 9 malignant cases1,10,12), we found 59 published case reports and series in the English-language literature. These tumors were reported within various age groups, but most commonly are reported in pediatric and young adult populations. Unlike intracranial schwannomas affecting cranial nerves, the intraparenchymal type shows no sexual predominance15), and has a low association with neurofibromatosis (only 4 of 66 cases were reported to have neurofibromatosis3,5,13)). Schwannomas can arise in any part of the brain parenchyma but 70% of them are supratentorial lesions, thus showing a predilection for the superficial cerebral cortex or a periventricular location15). Symptoms and clinical indications are usually related to the tumor location and size. In cases involving the superficial cerebral cortex, such as the one reported herein, seizure is one of the most common symptom presentations. Most lesions are reported to be small, less than 2 cm in diameter. However, there are cases that have undergone long indolent periods4,6,9,13). Eventually, such lesions may be more than 5 cm in diameter, and they present symptoms related to increased intracranial pressure, such as headache, vomiting, visual disturbance, hemiparesis, and change of mental status.
Certain radiographic characteristics of intracranial intraparenchymal schwannoma have been commonly reported, including intratumoral calcification (25%), cyst formation (>60%), mild to moderate peritumoral edema (>50%), and varying degrees of gadolinium enhancement of the solid tumor component8,15). However, many authors have indicated that there is no pathognomonic sign for intraparenchymal schwannoma, and that it is impossible to distinguish them from other tumors, such as pilocytic astrocytoma, ganglioglioma, pleomorphic xanthoastrocytoma, and dysembryoplastic neuroepithelial tumor, with radiographic evidence only. Large intraparenchymal schwannomas have been mistaken for meningioma or high-grade glioma11,14).
Our case appears to share many of the previously described typical features of intracerebral schwannoma. Young age, no known history of neurofibromatosis, seizure as an initial symptom presentation, small size mass located at the supratentorial cerebral cortex, peritumoral edema, and cyst are features that are commonly reported for intracerebral schwannoma. Preoperatively, we presumed the diagnosis of our case as pilocytic astrocytoma or pleomorphic xanthoastrocytoma because these tumors share the characteristics described above, and they have a higher incidence of occurrence. However, pathologic examination revealed that the tumor was a typical schwannoma, indicating our expectation was erroneous. Thus, we suggest that demographic, clinical, and radiologic findings are insufficient to derive an accurate diagnosis. We emphasize the importance of obtaining a surgical specimen in order to make proper diagnosis.
Intracerebral schwannoma is mostly benign and can be readily cured by surgery2,8,14,15). Typically, the tumor is well demarcated and can be easily dissected from brain parenchyme in most cases2). If the tumor is located at the superficial cerebral cortex, which is a common location for intracerebral schwannomas including our case, it can be removed completely without resulting in any neurological deficit.
Based on the 9 years of observations present for this case we suggest that this tumor type grows slowly, but even though the tumor may be small, it can produce a mass effect resulting in neurological symptoms through cyst formation. This may occur frequently because cyst formation near the tumor mass is a relatively common feature of schwannomas. Thus, considering the necessity of obtaining a correct diagnosis and a favorable surgical outcome, and because of patient deterioration that may result from simple observation-based diagnosis, early curative surgery is highly recommended when treating these tumors.
CONCLUSION
Intracerebral schwannomas are rare benign tumors that can be cured with complete surgical excision. Despite their rarity and the lack of pathognomonic radiologic findings, there are several demographic, clinical, and radiologic characteristics that can be helpful, but not definitive, when diagnosing intracerebral schwannoma. Considering the excellent surgical outcome and the risk of neurologic deterioration in the patient's near future, early curative surgery is highly recommended.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2012-0000996).