Comparison of Clinical and Radiologic Results between Expandable Cages and Titanium Mesh Cages for Thoracolumbar Burst Fracture
Article information
Abstract
Objective
A thoracolumbar burst fracture is usually unstable and can cause neurological deficits and angular deformity. Patients with unstable thoracolumbar burst fracture usually need surgery for decompression of the spinal canal, correction of the angular deformity, and stabilization of the spinal column. We compared two struts, titanium mesh cages (TMCs) and expandable cages.
Methods
33 patients, who underwent anterior thoracolumbar reconstruction using either TMCs (n=16) or expandable cages (n=17) between June 2000 and September 2011 were included in this study. Clinical outcome was measured by visual analogue scale (VAS), American Spinal Injury Association (ASIA) scale and Low Back Outcome Score (LBOS) for functional neurological evaluation. The Cobb angle, body height of the fractured vertebra, the operation time and amount of intra-operative bleeding were measured in both groups.
Results
In the expandable cage group, operation time and amount of intraoperative blood loss were lower than that in the TMC group. The mean VAS scores and LBOS in both groups were improved, but no significant difference. Cobb angle was corrected higher than that in expandable cage group from postoperative to the last follow-up. The change in Cobb angles between preoperative, postoperative, and the last follow-up did not show any significant difference. There was no difference in the subsidence of anterior body height between both groups.
Conclusion
There was no significant difference in the change in Cobb angles with an inter-group comparison, the expandable cage group showed better results in loss of kyphosis correction, operation time, and amount of intraoperative blood loss.
INTRODUCTION
A burst fracture is a compression fracture of anterior and middle columns of vertebral body and causes retropulsion of the posterior vertebral body fragment into the spinal canal by flexion-axial loading forces3). Nearly, 90% of all spinal fractures occur in the thoracolumbar region, and burst fractures represent 10% to 20% of such injuries. These fractures are usually unstable, and can cause neurological deficits and angular deformity. Therefore, patients with unstable thoracolumbar burst fractures usually need surgery. The goal of surgical treatment includes decompression of the spinal canal, restoration of the vertebral body height, correction of the angular deformity and stabilization of the spinal column. However, there are various surgical opinions regarding the optimal treatment. It is generally believed that anterior decompression and reconstruction with supplemental instrumentation are the most useful and effective methods, especially in unstable burst fractures with neurologic deficit.
In a lateral decubitus position, surgeons view the anterolateral aspect of the vertebral body for decompression. After corpectomy, bone graft or an implant is inserted to provide structural support, resulting in correction of angular deformity and restoration of body height. Traditionally, a structural autograft and allograft have been employed for bony reconstruction. However, high rate of donor site morbidity and pseudoarthrosis associated with the use of an allograft have been encountered, and the ability to correct the angular deformity was limited.
As an alternative, the titanium mesh cages (TMCs), having a hollow cavity for bone graft materials, can be used8,9,12,13,14,15,17). The TMCs are versatile in the diameter, length and shape. By selecting one such cage, the surgeon can achieve sagittal alignment. TMCs filled with bone graft materials can provide structural support and promote solid osseous union. Furthermore, TMCs maintain the stability of the spinal column and ensure long-term stability by supplemental instrumentation such as anterior or posterior percutaneous rod-screw system. However, the shape and length of titanium cages are fixed, and hence titanium cages of an appropriate length need to be designed before use. On the contrary, expandable cages can easily be fitted into the corpectomy defect and they can correct the sagittal imbalance. The surgeon simply needs to choose the cage and expands the cage until the appropriate height is achieved.
In this study, we retrospectively analyzed a series of 33 cases of unstable thoracolumbar burst fractures, which were treated with anterior decompression and reconstruction. And then, we compared the clinical and radiologic results between the two commonly used struts, TMCs and expandable cages, for anterior reconstruction after corpectomy.
MATERIALS AND METHODS
This study included 33 patients who underwent anterior thoracolumbar reconstruction using either TMCs (Pyramesh®, Medtronic Sofamor Danek, Memphis, TN, USA, n=16, group 1, between June 2000 and June 2007) or expandable cages (Synex ®, DePuy Synthes companies of Johnson & Johnson, Raynham, MA, USA; n=17, group 2, between April 2008 and September 2011) filled with bone graft materials between June 2000 and September 2011. Anterior or posterior percutaneous rod-screw system was also used to stabilize the affected segments in all cases. All patients were operated by one surgeon at a single institution.
All data including neurological improvement, subsidence, stability of the spinal column and sagittal balance were obtained from individual medical records and imaging studies before surgery, immediately after surgery, and at the last follow-up. For assessment of patients' clinical outcomes, visual analogue scale (VAS) for back pain, American Spinal Injury Association (ASIA) impairment scale on admission and at the last follow-up, and Low Back Outcome Score (LBOS) at post-operative 3 months, 6 months and the last follow-up were assessed. VAS is a horizontal line (100 mm in length) with word descriptors at each end, 0 (no pain) to 10 (worst pain imaginable). Although this score cannot be measured quantitatively, it is adequate enough to show the amount of pain that a patient feels ranges across a continuum from none to an extreme amount of pain. Neurological status was assessed by the ASIA standards : motor scores and ASIA impairment scale grade (A to E). Patients were considered to have an incomplete lesion if they had motor or sensory function in the sacral segments (sacral sparing). Neurological recovery was defined based on the improvement in motor scores and ASIA impairment scale grade. For measuring the functional outcome in a patient with low back pain, the authors used the LBOS. The total score of LBOS, the sum of points for all 13 parameters, ranges from 0 to 75 and the higher the score the better the patient's status. The operation time and amount of intra-operative bleeding were measured in both groups3,10).
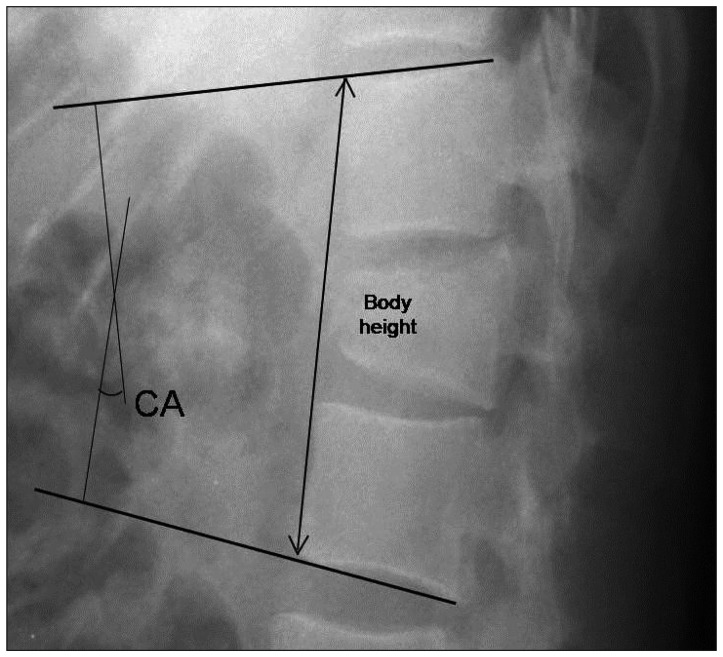
As radiologic parameters, the Cobb angle and body height of the fractured vertebra were evaluated. On a lateral plain radiograph, the kyphotic or lordotic angle was measured by calculating the angle of the intact endplate rostral and caudal to the fractured vertebra to evaluate the mean sagittal correction (Fig. 1)4). Also, the body height was measured between the anterior margins of the intact endplate rostral and caudal to the fractured vertebra to estimate subsidence before surgery and at the last follow-up using a lateral plain radiograph (Fig. 1). Computed tomography (CT) was performed in selected patients depending on the clinical indication.
Surgical technique
All patients were placed in a left lateral decubitus position on a radiolucent operating table. According to the level of the fractured vertebra, a retroperitoneal and/or a transpleural approach was performed. Usually, T12 or L1 burst fractures were approached using the retroperitoneal and transpleural routes, and burst fractures at L2 or below were approached via a retroperitoneal approach. The fractured vertebra and adjacent vertebrae were exposed and the segmental vessels were subsequently ligated. Corpectomy of the fractured vertebra, decompression of narrow spinal canal, and decortication of the endplates were performed for fitting a TMC or an expandable cage. Both cages were filled with autograft from the resected vertebral body.
Because of the fixed shape and length of TMCs, titanium cages of an appropriate length were designed before insertion. Because patient positioning caused thoracolumbar convexity on the same side of the operation, TMCs were inserted partially into the corpectomy defect. Then, TMCs were placed at a more central location using a mallet (Fig. 2).

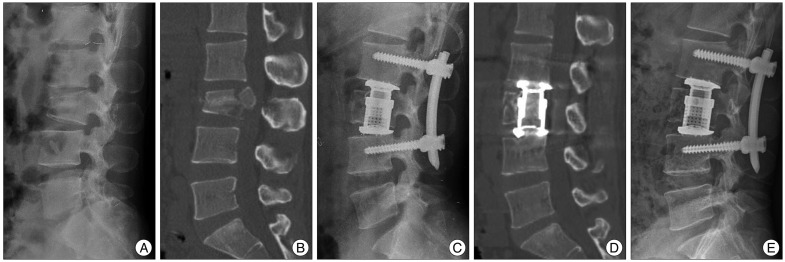
Case presentation 1. A : A 42-year-old woman sustained an L3 burst fracture after a fall. Lateral plain radiograph before the operation. B : Sagittal magnetic resonance imaging of the lumbar spine shows loss of height of the 3rd lumbar vertebra and bony retropulsion into the spinal canal. The Cobb angle was 7.71 degrees and anterior vertebral body height was 126.1 mm. C : With the use of a TMC, immediate post-operative lateral plain radiograph shows good implant placement with a Cobb angle of 10.21 degrees. D : One-year follow-up computed tomographic image shows solid fusion. E : On a lateral plain radiograph, -5.5 degrees of loss of kyphosis correction is observed.
Unlike TMCs, expandable cages can be lengthened by a built-in double-threaded system. Hence, expandable cages were located at the center of the corpectomy defect, and then they were expanded to the final height (Fig. 3).
Case presentation 2. A : A 30-year-old man sustained an L3 burst fracture after a traffic accident. Lateral plain radiograph before the operation. B : Sagittal computed tomographic image of the lumbar spine showing vertebral height loss and bony retropulsion into the spinal canal. The Cobb angle was -6.19 degrees and anterior vertebral body height was 114.3 mm. C : With the use of an expandable cage, immediate post-operative lateral plain radiograph shows good implant placement with a Cobb angle of 12.71 degrees. D : One year follow-up computed tomographic image shows solid fusion. E : One year follow-up computed tomographic image shows solid fusion.
After the placement of cages, all patients underwent supplemental instrumentation using anterior or posterior percutaneous rod-screw system. The configuration of the fixation was based on the degree of deformity and the experience of the surgeon in charge. The location of cages was checked by intraoperative fluoroscopy.
Statistical analysis
The clinical outcomes and radiographic parameters at the last follow-up were compared with those in the pre- and post-operative period. Data were analyzed using the SPSS program for Windows V18.0 (SPSS Inc., Chicago, IL, USA); the independent t-test, Mann-Whitney U test, and repeated measures ANOVA were used for analyses. Data are presented as mean±standard deviation. For all analyses, a p-value of <0.05 was considered statistically significant.
RESULTS
Group 1, in which TMCs were used, comprised of 9 males and 7 females, and the mean age was 39.9 years (range, 22--79 years). Group 2, in which expandable cages were used, consisted of 13 males and 4 females with the mean age of 48.1 years (range, 30--71 years). The average follow-up period was 32.4 months (range, 6--67 months) and 17.6 months (range, 6--44 months) in group 1 and group 2, respectively. The summary of the characteristics including the neurological status of 33 patients on admission is provided in Table 1.
Clinical outcomes
The mean VAS scores of group 1 and 2 were 6.89±1.56 and 7.63±1.55 respectively before surgery, and 2.55±1.31 and 2.09±0.95 respectively at the last follow-up. The mean LBOS in the group 1 was 42.85±13.36 at 3 months, 47.75±15.81 at 6 months, and 53.51±16.14 at the last follow-up. In group 2, the mean LBOS was 45.64±17.85, 52.31±12.34, and 55.42±11.74 at 3 months, at 6 months, and at the last follow-up, respectively. Although the mean VAS scores and LBOS were improved during the follow-up period, there was no statistically significant difference between the two groups (p=0.150) (Table 2).
Regarding the neurological status, in group 1, neurological status was improved in 10 patients and it remained unchanged in 6 patients including 3 patients with ASIA scale E. In group 2, 6 patients showed neurological improvement, and neurological status remained unchanged in 11 patients. Three of these 11 patients had an ASIA scale E on admission and at the last follow-up. None of the patients in both groups had neurologic aggravation (Table 3).
The average operation time in group 1 and 2 was 332.51±61.04 min and 250.32±54.42 min, respectively, and the amount of intraoperative blood loss was 1408.83±523.63 cc and 823.54±577.51 cc, respectively (Table 2). In group 1, the operation time (p<0.001), and the amount of intraoperative blood loss (p=0.005) were significantly higher than those in group 2. However, there was no significant difference in age (p=0.150) and sex (p=0.575) between both groups (Table 1).
Radiologic outcomes
The average preoperative Cobb angle in group 1 and 2 was -11.94±11.91 degrees and -18.07±9.85 degrees, respectively, which was significantly corrected to -3.13±11.55 degrees and -5.98±8.56 degrees immediately after surgery (p<0.001). At the last follow-up, the Cobb's angle in group 1 and 2 was mildly aggravated to -9.13±12.48 and -7.81±10.62 degrees, respectively (p<0.001). However, the correction in Cobb angles at the last follow-up was well maintained compared with Cobb angles in the preoperative period in both groups (p<0.001). In the postoperative period, using either TMCs or expandable cages, significantly affected the change in Cobb angles with an intra-group comparison. However, with an inter-group comparison, the change in Cobb angles measured at the preoperative, immediate postoperative, and the last follow-up period did not show a statistically significant difference (p=0.485) between the two groups (Fig. 4).

The change in the Cobb angle between the TMC and expandable cage groups in the preoperative, postoperative and last follow-up periods. TMC : titanium mesh cage.
The mean loss of kyphosis correction between immediate postoperative and last follow-up was -5.99±5.51 and -1.82±3.22 degrees in group 1 and 2, respectively. The average loss of kyphosis correction in group 1 was significantly higher than that in group 2 during the follow-up period with a statistical significance (p=0.012) (Table 2). The subsidence of anterior body height was 7.99±2.23 cm and 9.85±1.60 cm in group 1 and 2, respectively, and there was no significant difference (p=0.056) (Table 2).
DISCUSSION
On the basis of the results of our study, anterior reconstruction surgery using either TMCs or expandable cages with supplemental instrumentation was effective for the initial angular deformity correction. Although there was no statistically significant difference among the preoperative, immediate postoperative, and last follow-up Cobb angles with an inter-group comparison, the loss of kyphosis correction in the expandable cage group was significantly less than that in the TMC group during the follow-up period. Furthermore, in the expandable cage group, operation time and amount of intra-operative blood loss were significantly lower than those in the TMC group.
Decompression of the spinal canal, achieving rigid fixation, correcting the sagittal imbalance, achieving a solid fusion and maintenance of spinal alignment are the purposes of surgical treatment of thoracolumbar burst fracture. In spite of good immediate postoperative outcomes, angular deformity may tend to worsen during the follow-up period. This seems to be due to the progressive loss of vertebral body height and the alteration in biomechanical characteristics of the adjacent motion segments and soft tissues. Therefore, some authors suggested that the primary surgical method must be selected based on the severity of the initial angular deformity10,14,18).
The posterior surgical approach is well known for its various advantages, such as safety, wide surgical field of view and less technically demanding procedure. In the posterior approach, collateral injuries to visceral, pulmonary and vascular organs can be avoided. Therefore, posterior decompression of the spinal canal and short-segment fixation without anterior reconstruction is commonly performed. However, in spite of its advantages, the posterior surgical approach has several limitations. Posterior short-segment fusion has been found to be associated with a construction failure with an increase in kyphosis. Especially, the long-segment fusion (involving two levels above and two levels below) may lead to a reduction in the range of spinal motion. Meanwhile, the anterior approach can cause direct decompression of the spinal canal and reconstruction of the anterior spinal column. In cases with an intact posterior longitudinal ligament and posterior spinal column, the anterior approach may be applied. However, the operative risk for the anterior approach is higher than that for the posterior approach1,6,11,16).
Traditionally, polymethyl methacrylate, autologous or allogenic bone grafts and mesh vertebral cages have been used for anterior reconstruction after corpectomy. Bone graft, such as an autograft or allograft, was the gold standard for spinal fusion. However, complications such as graft collapse and migration were observed. Also, additional time was required to design a graft of an appropriate size and shape of graft for the adequate bedside. Therefore, cages were introduced for vertebral body replacement in spinal fusion2,6,7,17,19).
Some studies have evaluated patients with thoracolumbar burst fractures associated with kyphosis after surgery using the anterior approach. Dvorak et al.4) studied 26 cases with thoracolumbar burst fractures, in which TMC with an autograft was used and supplemented with an anterior plate; the average preoperative kyphosis was 25.4±18.4 degrees, after surgery, it improved to 7.5±15.3 degrees, and on follow-up evaluation, kyphosis was 10.4±13.2 degrees. Kaneda et al.9) reported that the kyphosis correction using a titanium mesh and a plate ranged between 6 and 10 degrees at the last follow-up. Uchida et al.17) reported that using an expandable cage appears to aid in the restoration of the physiologic thoracolumbar curve.
Eleraky et al.6) reported that expandable cages enabled greater kyphosis correction immediately after surgery and a similar degree of correction was observed at the final follow-up. They concluded that correction of the kyphotic deformity was much easier using an expandable cage, and it can be easily inserted into the corpectomy defect. On the other hand, non-expandable cages like TMCs, must be trimmed to an adequate size and then they are malleted into the defect5). Contrarily, Lau et al.13) reported that expandable cages were associated with higher risk and greater magnitude of subsidence than that of static cages. However, they suggested that this result might be due to the technical aspects and there was no feedback or objective gauge to monitor how much force was applied to the defective vertebral body.
Selecting a wrong size of TMC may be associated with a greater chance of cage migration, followed by delayed progression of angular deformity recurrence. Moreover, a sharp edge of TMC and inappropriate fitting of the cage may lead to additional vertebral injury and angular deformity. In contrast, easy cage insertion and convenience of designing an expandable cage would enable to reduce the operation time and the amount of intra-operative blood loss.
There are several limitations to this study, such as the small number of patients, relatively short-term follow-up period, and the difference in the follow-up period between the two groups. Also, the author used TMCs between June 2000 and June 2007 and expandable cages between April 2008 and September 2011. Thus, there could be some differences in surgeon's experience and workmanship between the two groups. Moreover, methods of supplemental instrumentation were different in both groups. These limitations might have influenced the clinical and radiologic outcomes.
CONCLUSION
Clinically, anterior reconstruction with either TMCs or expandable cages after corpectomy can be a safe and reliable surgical treatment option for thoracolumbar and lumbar burst fractures. However, anterior reconstruction using an expandable cage is associated with a lower loss of kyphosis correction with minimal subsidence. The results of this study suggest that surgery using the expandable cage may be a more feasible treatment option for thoracolumbar and lumbar burst fractures.