Large Intracranial Aneurysm after Transsphenoidal Surgery for Pituitary Macroadenoma
Article information
Abstract
Uncontrolled cerebrospinal fluid (CSF) leakage after transsphenoidal surgery (TSS) for pituitary adenoma can lead to meningitis. Intracranial mycotic pseudoaneurysm is a rare complication in central nervous system infection. Large single pseudoaneurysm is more uncommon. Most mycotic aneurysms occur due to endocarditis. The present patient had no heart problem and was infected by CSF leakage after transsphenoidal surgery. We present a case of large ruptured mycotic pseudoaneurysm as a complication of cerebral infection after TSS for pituitary macroadenoma.
INTRODUCTION
Endoscopic transsphenoidal surgery for pituitary adenoma has been recognized as an alternative intracranial approach. It is less invasive than conventional surgery. Cerebrospinal fluid (CSF) leakage is an adverse event during surgery that can cause an infection of the central nervous system (CNS). Meningitis is one of the critical complications after transsphenoidal pituitary adenoma removal, especially in cases of CSF leakage2). Prolonged and uncontrolled meningitis or cerebral infection may develop mycotic or infectious cerebral pseudoaneurysms.
Mycotic pseudoaneurysm is a rare clinical entity comprising less than 0.7% of intracranial aneurysms6). Mycotic cerebral pseudoaneurysm is related with infections, especially infectious endocarditis1). It is prone to rupture and presents a poor clinical outcome and high mortality13). Mycotic aneurysms are generally characterized as small size, multiple lesions and vulnerability to rupture.
We present a case of large rupture mycotic pseudoaneurysm due to cerebral infection after transsphenoidal surgery (TSS) for pituitary macroadenoma.
CASE REPORT
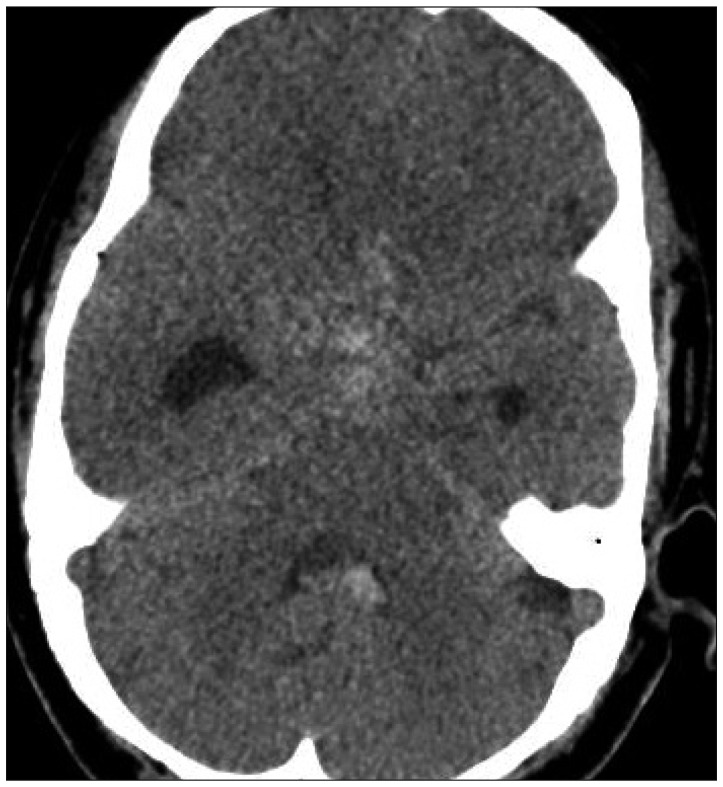
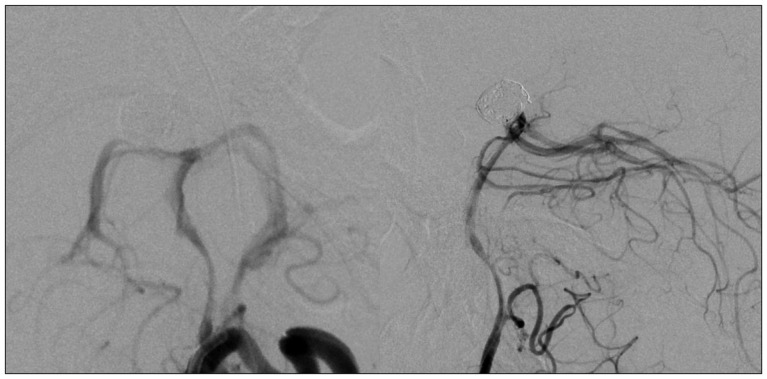
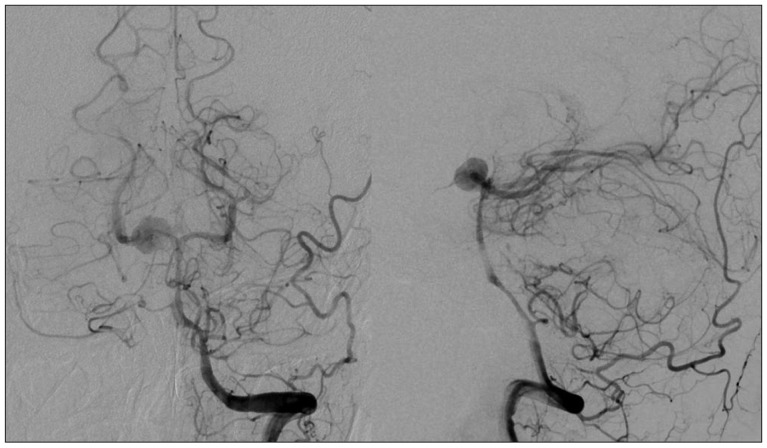
A 44-year-old woman who presented with a suprasellar mass was referred from a local clinic to our department. She had no past medical history and familial history. Brain MRI showed suprasella mass (1.4×1.0×1.6 cm) with heterogenous enhancement and fluid-fluid level, implying focal hemorrhage (Fig. 1). Preoperative hormone study showed normal findings. Preoperative visual field test showed no abnormal finding and transthoracic echocardiography revealed no definite abnormality in heart function and morphology. We planned transsphenoidal endoscopic surgery under impression of pituitary macroadenoma with focal hemorrhage. We performed endoscopic surgery and completely removed the mass. Just after removal of the mass, there was no problematic event. However, during fat packing, sudden small arterial bleeding from the site of tumor removal occurred. We tried but failed to control bleeding by electrocautery. We packed the site with small cotton soaked in epinephrine solution to control bleeding. After compression of the bleeding focus, bleeding was resolved and skull base reconstruction was performed by bone chip, fibrin glue and local fat tissue. We confirmed no CSF leakage. On the first postoperative day, CSF leakage from nasal cavity was suspected. We decided to perform lumbar CSF drainage. During the first week after surgery, mild fever was persistent and mild CSF leakage was noted. We did not consider skull base repair because CSF leakage was decreased gradually. On postoperative day 7, the mild fever changed to high fever (>38℃) and CSF leakage increased. We made a decision to perform skull base repair surgery. CSF pooling and leakage from a sellar floor defect was confirmed. We harvested septal cartilage and vomer from the nasal cavity and completely reconstructed the sellar floor defect. At the end of surgery, there was no more CSF leakage. After the second operation, the CSF profile (protein 99.4 mg/dL, glucose 35 mg/dL) indicated a suspicion of CNS infection and antibiotics were changed to treat meningitis. Culture study did not present infected organism, but clinical symptom and CSF profiles supported a diagnosis of cerebral infection. Fever was persistent and CSF profiles were slightly improved after the second operation. However, the patient's condition was ingravescent, and finally involved sepsis. On the seventh postoperative day from second surgery, the patient presented fixed pupil light reflex (3 mm/4 mm) and sudden decreased mentality. We immediately performed a brain computed tomography (CT) and CT angiography that revealed subarachnoid hemorrhage (SAH) and intra-ventricular hemorrhage (Fig. 2). The patient underwent emergency cerebral angiography, which revealed a large pseudoaneurysm at the right posterior cerebral artery (PCA) (Fig. 3). We did not perform preoperative cerebral angiography but preoperative brain MRI did not show such a large aneurysm at same site (Fig. 1). Emergency coil embolization was carried out by using commercially available detachable coils (1 GDCs; Boston Scientific/5 Target 360 soft coils, Boston Scientific) (Fig. 4). After coil embolization, we treated cerebral infection by antibiotics and tried prevent cerebral vasospasm. Unfortunately, intractable seizure developed and the patient died on postoperative day 12 from the second surgery.
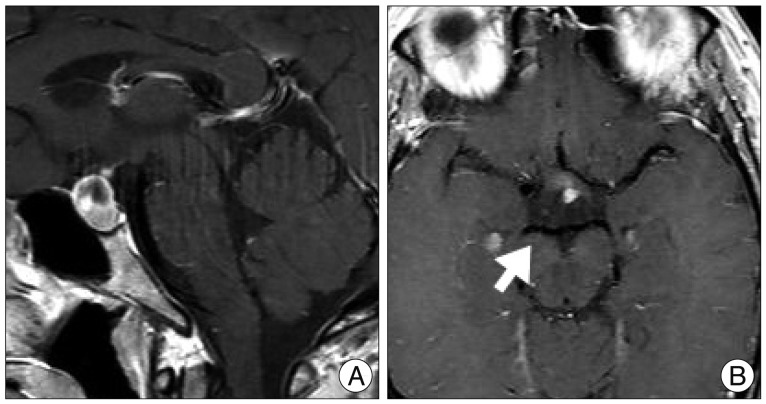
A : Preoperative sellar MRI (sagittal view) shows suprasella mass (1.4×1.0×1.6 cm) with heterogenous enhancement and fluid-fluid level, implying focal hemorrhage. B : Preoperative enhanced MRI (axial view) shows no evidence of right posterior cerebral artery aneurysm (arrow).
Conventional cerebral angiography (anteroposterior and lateral view) shows a large pseudoaneurysm at right posterior cerebral artery.
DISCUSSION
Since introduction of endoscopic TSS for pituitary adenoma, it has become a useful neurosurgical modality4). Complications include CSF leakage, nasal vascular complication, infection and endocrine complication2). CSF leakage can occur during excision of the sellar floor or after removal of tumor. In case of clinical suspicion of CSF leakage, lumbar drainage can be attempted to stop leakage. Prolonged CSF leakage may result in ascending infection to the meninges or cerebrum. The rate of infectious complications ranges from 0.5% to 5.7%2). As a consequence, uncontrolled infectious condition of CNS infection causes infectious intracranial pseudoaneurysms8). Our patient presented CSF leakage after surgery but leakage decreased after lumbar drainage. So, we decided observe for a while. However, one week from the surgery CSF leakage increased, prompting the decision for reconstruction surgery.
Infectious or mycotic intracranial aneurysms are rare, but may prove lethal when rupture occurs8). Intracranial hemorrhage due to infectious pseudoaneurysm is uncommon but presents a high mortality near 80%13). In 1968, Nakata et al.15) documented using an experimental model that bacterial blood stream infection can cause formation of bacterial aneurysm. In our case, there were three possible mechanisms of pseudoaneurysm formation. First, mechanical or iatrogenic direct injury of vessel can causes a pseudoaneurysm. It is most common cause of pseudoaneurysm. It mainly arises at cavernous segment of internal carotid artery. However, as the right PCA is too far from the sellar, so this possibility was very low in our patient. Second, CNS infection is the second most common cause of intracranial pseudoaneurysm5). Our patient suffered from a CNS infection due to CSF leakage. This mechanism is the likeliest in this case. Lastly, chemical irritation around the operation site by hemostatic material, such as epinephrine solution or glue, is possible, but is unlikely for the same reason as the iatrogenic injury. Septic emboli cause the earliest changes in the adventitial layer and diffuse to the media. With time, necrosis or rupture of arterial wall, eventually shape a pseudoaneurysm14,15). Mortality of mycotic pseudoaneurysm even with treatment involving surgery and antibiotics ranges from 7% to 61%5). Meningitis and SAH also may develop in mycotic pseudoaneurysm15). In our cases, CSF culture study did not show any infectious organism. Sometimes, CSF study does not help to identify organism in infectious aneurysm. Pruitt et al.16) reported only 16% of infectious aneurysm patients group showed positive CSF cultures.
Mycotic pseudoaneurysms are pathophysiologically false aneurysms that have a weak wall, so compact packing in aneurysm sac increase rupture risk during endovascular surgery12). Mycotic pseudoaneurysms arise from necrosis of vessel wall by bacterial emboli5). Growth of pseudoaneurysms is unforeseeable and they are sometimes regressed or ruptured. Antibiotics treated mycotic pseudoaneurysms evolve to several situations as follows resolution itself, enlargement, new aneurysm formation and rupture5). Mycotic pseudoaneurysms exhibit high risk of rupture and its rupture risk increases by aneurysm size7).
Basic treatment of mycotic pseudoaneurysms is occlusion, which is intractable with antibiotic treatment. Some mycotic pseudoaneurysms may be treated by medical treatment alone3). In our case, occlusion of pseudoaneurysm was inevitable because spontaneous SAH was already developed by rupture of pseudoaneurysm and antibiotics had been used to treat cerebral infection since surgery. Recently, endovascular treatment has gained more favor than open surgery19). Several reports and study groups reported the safety and efficacy of endovascular treatment for mycotic pseudoaneurysms18,19).
Endovascular treatment of mycotic pseudoaneurysm is second to none in patients who do not present intracranial hematoma, which produces a mass effect6). Chapot et al.5) reported that endovascular treatment appears to improve outcome concerning the lethal condition of ruptured pseudoaneurysm. Mycotic pseudoaneurysms easily rupture during surgical ligation or clipping because the aneurysm wall is very weak12). Therefore, occlusion of parent artery with endovascular technique can sometimes be justified when distal flows are compensated by other arterial circulation9).
One of the serious complications during endoscopic transsphenoidal surgery is iatrogenic vascular injury. Complication rate of vascular injury during transsphenoidal surgery for pituitary adenoma is reported as approximately 1%10). Injury of internal carotid artery (ICA) during transsphenoidal surgery of pituitary adenoma may result in catastrophic situation including death. This event may bring on uncontrolled arterial bleeding, vasospasm, delayed pseudoaneurysm and thrombosis of ICA5). Several predisposing factors to ICA injury have been introduced including large invasive adenoma, transsphenoidal surgery, radiation treatment, prolonged dopamine agonist and aggressive surgical attempt to remove whole pituitary mass17). The most common injury of vessel during TSS is cavernous ICA17). Several reports have described iatrogenic pseudoaneurysms during TSS11,17). In our case, the iatrogenic possibility of pseudoaneurysm was low because the aneurysm originated from the right PCA.
CONCLUSION
CSF leakage after transsphenoidal endoscopic surgery for pituitary adenoma should not be ignored because it may bring a regretful situation. CNS infection from CSF leakage can cause many serious complications and evolve mycotic pseudoaneuryms. Large cerebral pseudoaneurysm is a rare complication after TSS and can bring high mortality. As shown in this report, physicians should aware the possibility of pseudoaneurysm after CNS infection. Early suspicion and diagnosis in this clinical seeting is mandatory because of its possible morbid outcome when delayed. Endovascular treatment might be primary modality in the treatment of mycotic pseudoaneurysm.
Acknowledgements
This work was supported by Dankook University research fund in 2011.