Effect of Discontinuation of Anticoagulation in Patients with Intracranial Hemorrhage at High Thromboembolic Risk
Article information
Abstract
Objective
There was no abundance of data on the use of anticoagulant in patients with previous high risk of thromboembolic conditions under a newly developed intracranial hemorrhage in Korean society. The purpose of this study was to evaluate the safety of discontinuance and suggest the proper time period for discontinuance of anticoagulant among these patients.
Methods
We reviewed the medical records of 19 patients who took anticoagulant because of thromboembolic problems and were admitted to our department with newly developed anticoagulation associated intracranial hemorrhage (AAICH), and stopped taking medicine due to concern of rebleeding from January 2008 to December 2012. Analysis of the incidence of thromboembolic complications and proper withdrawal time of anticoagulant was performed using the Kaplan-Meier method.
Results
Our patients showed high risk for thromboembolic complication. The CHA2DS2-VASc score ranged from two to five. Thromboembolic complication occurred in eight (42.1%) out of 19 patients without restarting anticoagulant since the initial hemorrhage. Among them, three patients (37.5%) died from direct thromboembolic complications. Mean time to outbreak of thromboembolic complication was 21.38±14.89 days (range, 8-56 days). The probability of thromboembolic complications at 7, 14, and 30 days since cessation of anticoagulation was 0.00, 10.53, and 38.49%, respectively.
Conclusion
Short term discontinuance of anticoagulant within seven days in patients with AAICH who are at high embolic risk (CHA2DS2-VASc score >2) appears to be relatively safe in Korean people. However, prolonged cessation (more than seven days) may result in increased incidence of catastrophic thromboembolic complications.
INTRODUCTION
Long term use of chronic oral anticoagulants can be complicated by anticoagulation associated intracranial hemorrhage (AAICH), the most devastating complication of anticoagulant therapy. Among patients who received anticoagulant for management of thromboembolic problems, intracranial hemorrhages led to approximately 90% of deaths and major disability in survivors5). AAICH is an urgent medical condition requiring prompt reversal of anticoagulation. Rapid reversal is essential for prevention of enlargement of the AAICH, which could result in brain herniation. On the other hand, reversal and discontinuance of anticoagulant can result in development of several kinds of side effects, such as thromboembolic complication. The risk and incidence of thromboembolic complication after cessation of anticoagulation in Korean society have not been accurately reported; in addition, there is no consensus regarding the proper period for withholding anticoagulant in the case of these patients. Based on results of other studies, discontinuation for seven to 10 days appears to be safe2). However, this has limitation as a proper guideline because these data were reported from small western retrospective series3,6,11). In this study, we discussed reasonable access for management of patients with AAICH who are at a high thromboembolic risk in the view of using an anticoagulant.
MATERIALS AND METHODS
Patient selection
From January 2008 to December 2012, 47 patients with warfarin associated intracranial hemorrhage were admitted to a single neurological institution. Eleven patients (23.4%) died due to an initial massive hematoma and were excluded from this study. Of the remaining 36 patients, 19 patients were not restarted with anticoagulant due to high risk of rebleeding and 17 patients were restarted with anticoagulant at a different time depending on each patient's condition. Restarting of drug was decided after consultation with the doctor who treated the patients due to the underlying disease. However, they did not give a clear answer regarding when the drug should be restarted after hemorrhage. Opinion regarding reuse of anticoagulant differs significantly depending on the physician. If the rebleeding risk was greater than the thromboembolic risk, anticoagulants were not restarted. For these reasons, anticoagulant was not started for 19 patients. Underlying disease to get oral anticoagulant, presenting hemorrhage type, thromboembolic risk factors, event day international normalized ratio (INR), prothrombin time (PT), and thromboembolic complication after cessation of anticoagulation were investigated.
Statistical analysis
Data analysis was performed retrospectively using the Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were performed for determination of the influence of clinical factors on development of thromboembolic events in the non-restarted anticoagulant group. The Kaplan-Meier method was used to assess the incidence and probability of thromboembolic events in patients with passage of time.
CHA2DS2-VASc score
Thromboembolic risk is largely dependent on the patient's condition itself. We need to identify the exact degree of thromboembolic risk in the patients' group in order to obtain objective validity when comparing the results with those of other authors. In this study, we used CHA2DS2-VASc score. The CHA2DS2-VASc [congestive heart failure/left ventricular dysfunction, hypertension, age ≥75 (double), diabetes, stroke (double)-vascular disease, age 65-74, and sex category (female)] has been used in multiple cohorts and is better at identifying patients with true risk. The score is inclusive of the most common risk factors for stroke in everyday clinical practice4,8).
RESULTS
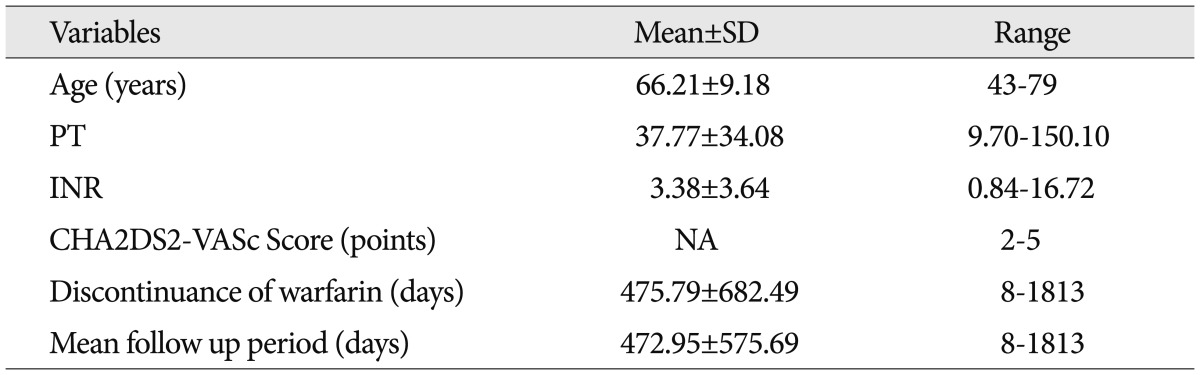
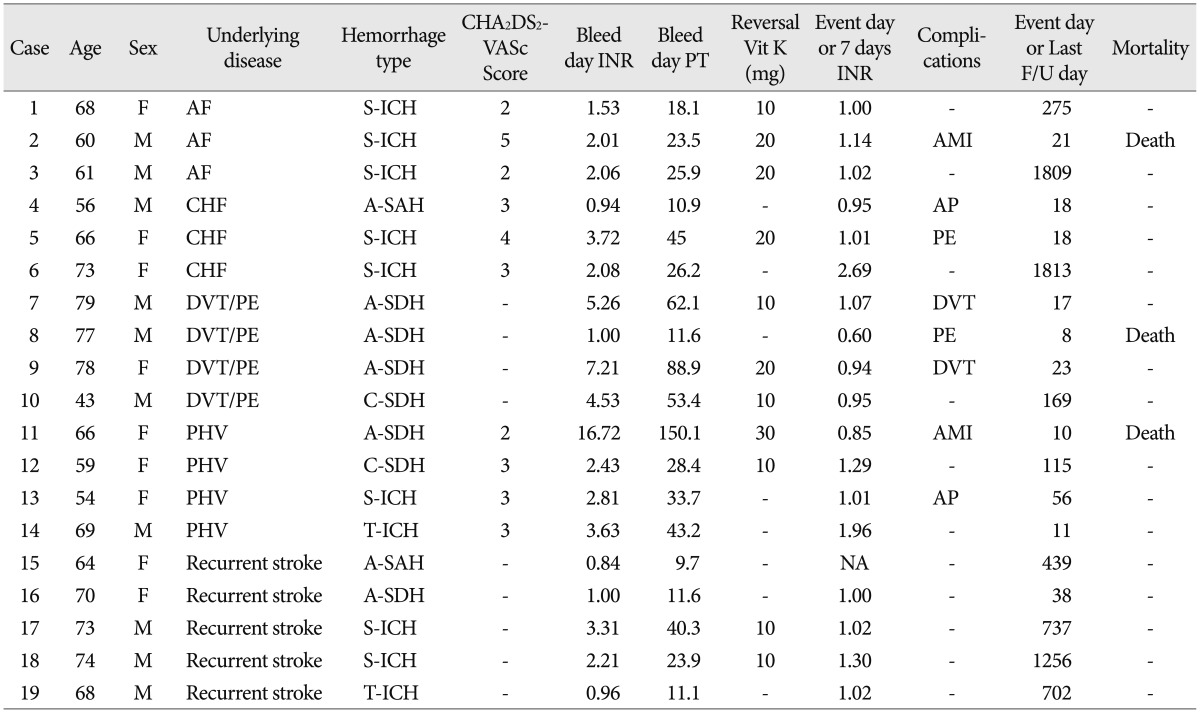
The demographic and clinical characteristics of 19 patients in whom anticoagulants were not started are shown in Table 1 and 2. The most common type of AAICH was spontaneous hemorrhage, which presented in eight cases (42.1%). Acute subdural hematoma was the next main type of AAICH in five cases (21.1%). The mean PT was 37.77±34.08 seconds (range, 9.70-150.10 seconds). The thromboembolic risk of patients was calculated by the CHA2DS2-VASc score8). All patients presented with CHA2DS2-VASc scores of 2 or more than 2 points. The mean discontinuance time of the anticoagulant was 475.79±682.49 days (range, 8-1813 days).
There were eight cases (42.1%) with thromboembolic complications, three of which (37.5%) resulted in death. Two deaths were caused by sudden cardiac arrest, which was suspected with acute myocardial infarction, and another patient died of pulmonary thromboembolism. Among the survivors, deep venous thrombosis followed by intravenous filter insertion occurred in two cases and another two patients presented with angina pectoris. And non-fetal pulmonary embolism occurred in one case. The INR score at the time of occurrence of thromboembolic complications was 0.95±0.16. Mean time to occurrence of thromboembolic complication was 21.38±14.89 days (range, 8-56 days). The probabilities of having thromboembolic complications at 7, 14, and 30 days following cessation of warfarin therapy were 0.0% (95% confidence interval, 0.00-20.92%), 10.53% (95% confidence interval, 1.84-34.54%), and 38.49% (95% confidence interval, 18.42-62.85%), respectively (Fig. 1). By seven days, there was no thromboembolic complication or death. After seven days, thromboembolic complications showed a rapid increase. The INR showed a gradual decrease to the normal range, and then thromboembolic complications started to occur after a few days. From the result, we found that all thromboembolic complications and death occurred after seven days in all cases, and most of these thromboembolic complications tended to be concentrated between one and two weeks.

Kaplan-Meier thromboembolic complication free survival estimate for patients with anticoagulants associated intracranial hemorrhage without resuming anticoagulants. The probability of thromboembolic complications after cessation of anticoagulation at 7, 14, and 30 days was 0.00, 10.53, and 38.49%, respectively.
DISCUSSION
This study provided the longest follow up data on discontinuance of anticoagulant in patients presenting with AAICH who have been at high thromboembolic risk estimated by CHA2DS2-VASc score in Korean society.
This report presented several important findings. First, discontinuance of the anticoagulant is common in AAICH patients in the actual clinical field. Proper restart of warfarin treatment after AAICH was not prescribed for almost half of patients (47.2%, 17/36). AAICH induced mortality is very high and survivors from initial neurological damage are faced with the decision of whether or not to restart anticoagulan3,5). However, proper use of anticoagulant in patients with AAICH has not yet been established, and guidelines could not be obtained from a limited number of previous retrospective series3,6,11). According to American Heart Association recommendations, in patients with a very high risk of thromboembolism in whom restarting warfarin is considered, warfarin therapy may be restarted at seven to 10 days since onset of the original hemorrhage2). In this study, only nearly half of patients were prescribed anticoagulant because of hemorrhagic risk. The consensus of the resumption of anticoagulant in AAICH patients is not clear in our institution. Decisions regarding to the starting time of anticoagulant were differed significantly among physicians due to the fact. And available data for determination of the optimal timing and dosage for restarting anticoagulation following an episode of AAICH are inadequate.
Second, in this study, short duration (within 7 days) discontinuance of anticoagulants was relatively safe, similar to the result of a previous study series. Kawamata et al.7) found no cases of ischemic stroke when warfarin treatment was discontinued for three days in 27 patients with AAICH. Tinker et al.10) retrospectively reviewed the risk of discontinuing warfarin therapy in 159 patients with mechanical heart valves undergoing elective surgery; none of the patients had in-hospital thromboembolic complications. Ananthasubramaniam et al.1) also reported that no thromboembolic complications occurred in 24 patients with prosthetic heart valves after withholding anticoagulant for an average of 15 days. Like these previous studies, in this study, there was also no occurrence of thromboembolic complications within seven days since AAICH.
The third finding of this study is as follows. Although a temporary withdrawal of anticoagulants within seven days may be safe, prolonged cessation could lead to catastrophic results. We found that thromboembolic complications begin to occur after seven days and the incidence shows a rapid increase around 14 days later. Two complications occurred within two weeks. However, additional events occurred after two weeks. Overall occurrence of thromboembolic complications was 42.1% (8/19), higher than that reported in previous series1). The reasons for this phenomenon can be explained as follows. First, the time for withdrawal of anticoagulant was long, compared to the previous reports. In previous studies1), anticoagulant was restarted within at least two weeks after a hemorrhagic event. The INR in patients with thromboembolic complication showed a gradual reduction and it fell below the normal range on ictus days. Because the thromboembolic risk was accumulated after cessation of anticoagulants, the possibility of thromboembolic event also increased. Second, the patients belonged to a very high risk group for occurrence of thromboembolic events. The CHA2DS2-VASc score has been used in multiple cohorts for measurement of severity and for better identification of patients with true risk4). The score includes the most common risk factors related to stroke in everyday clinical practice. Among patients with CHA2DS2-VASc score=2, the incidence of thromboembolic complication in one year is 2.69% and the rate increased by 3.20% at a score of 3 points. For prevention of thromboembolism, anticoagulants are recommended for patients with a score of more than 2 points9). In this study, the CHA2DS2-VASc score was more than 2 points for all patients (2 points in three cases, 3 points in five, and 4 and 5 points in one case each), indicating that the patient has a high underlying thromboembolic risk. Otherwise, previous reports did not objectively demonstrate the patients' underlying thromboembolic risk, causing difficulty in comparison and analysis of results between studies.
This study included only 19 cases. The limitation of this study is the retrospective design with a very small number of cases. However, the incidence of patients who did not restart anticoagulation after AAICH was very low. Over the past five years, we were only able to collect 19 cases. Previous studies also included small case series with retrospective review3,6,11). This study is expected to have worth as the first study of Korean people.
CONCLUSION
Discontinuance of anticoagulant within seven days presented a relatively low incidence of thromboembolic complications in patients with AAICH at a high thromboembolic risk (CHA2DS2-VASc score >2), and withdrawal of more than seven days may increase the risk of devastating results. We think that short term discontinuance of anticoagulant limited within seven days appears to be safe in Korean people. Unfortunately, this study was conducted retrospectively in a small number of cases of AAICH and has several limitations to understanding. For a more reasonable and acceptable guideline on the use of anticoagulant in patients with AAICH, conduct of further studies will be required.