A Retrospective Analysis of Ventriculoperitoneal Shunt Revision Cases of a Single Institute
Article information
Abstract
Objective
Ventriculoperitoneal (VP) shunt complication is a major obstacle in the management of hydrocephalus. To study the differences of VP shunt complications between children and adults, we analyzed shunt revision surgery performed at our hospital during the past 10 years.
Methods
Patients who had undergone shunt revision surgery from January 2001 to December 2010 were evaluated retrospectively by chart review about age distribution, etiology of hydrocephalus, and causes of revision. Patients were grouped into below and above 20 years old.
Results
Among 528 cases of VP shunt surgery performed in our hospital over 10 years, 146 (27.7%) were revision surgery. Infection and obstruction were the most common causes of revision. Fifty-one patients were operated on within 1 month after original VP shunt surgery. Thirty-six of 46 infection cases were operated before 6 months after the initial VP shunt. Incidence of shunt catheter fracture was higher in younger patients compared to older. Two of 8 fractured catheters in the younger group were due to calcification and degradation of shunt catheters with fibrous adhesion to surrounding tissue.
Conclusion
The complications of VP shunts were different between children and adults. The incidence of shunt catheter fracture was higher in younger patients. Degradation of shunt catheter associated with surrounding tissue calcification could be one of the reasons of the difference in facture rates.
INTRODUCTION
The ventriculoperitoneal (VP) shunt has been a main treatment modality for hydrocephalus since John Holter invented the shunt valve in 19592). However, complications are still a major obstacle in the management of this relatively common neurosurgical disease23). Continued development of the shunt device has provided significant improvement to the outcome of hydrocephalus patients. Recently, adjustable pressure valves and antibiotic-impregnated shunt catheters have been used to continue the trend in catheter development for better outcomes10151718192326).
The VP shunt has specific features that have to perform across a wide range of patient ages, from premature to very old patients, and has to be maintained during a patient's whole life23). Because about half of hydrocephalus patients involve children2829), physicians responsible for the disease should be aware of the management of the hydrocephalus both in children and adults. Varieties of complication develop in patients, and they differ according to a patent's age24). Issues due to transition of patients from pediatric to adult as the patients grow were studied by many authors2425283437). However, it is hard to find studies about the differences of shunt complications according to patient's age. We analyzed VP shunt complications at our hospital to find their clinical features and elucidate differences in complication patterns according to a patient's age.
MATERIALS AND METHODS
Our hospital is the third referred university hospital with active pediatric neurosurgery. Patients who had VP shunt revision surgery at our institution from January 2001 to December 2010 were retrospectively reviewed for the cause of hydrocephalus, operation history, complications, and the cause of revision. Other shunt surgeries, such as cystoperitoneal or subduroperitoneal shunts, were not included.
Patient diagnoses were divided into the following etiologies : trauma, spontaneous subarachnoid hemorrhage, tumor, germinal matrix hemorrhage, idiopathic normal pressure hydrocephalus, congenital anomaly, and other. Causes of revision were divided into infection, malposition, obstruction, catheter fracture, slit ventricle syndrome, useless shunt, and other. The causes of shunt revision were analyzed according to time interval between original shunt surgery and revision and the age of the patients. Patients were grouped into below and above 20 years old.
Statistical analysis was done using SPSS (Ver 19, Chicago, IL, USA). The chi-square test was used to evaluate statistical significance in group differences, and a p-value of less than 0.05 was regarded as statistically significant.
RESULTS
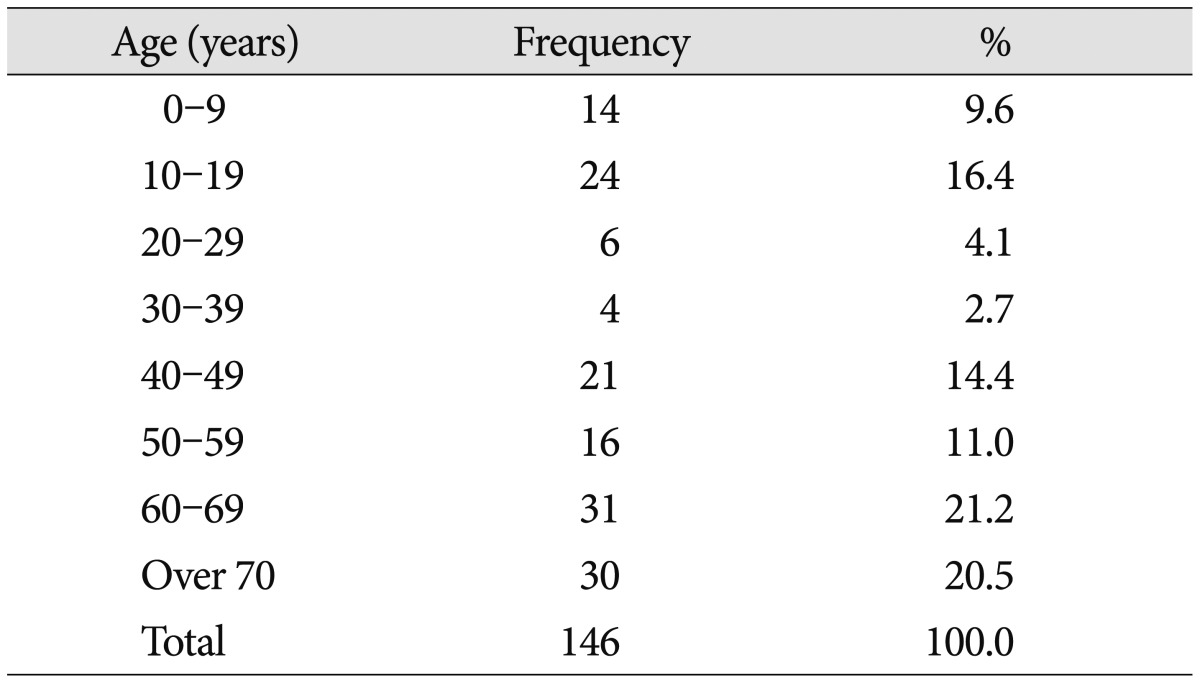
The total number of VP shunts performed during the 10-year period from January 2001 to December 2010 was 528. Among them, 146 cases (27.7%) were revision surgery and were included in this study. There were 73 males and 73 females. The mean age of the patients was 46.5 years (median 52 years, ranging from 1 month to 85-years). Thirty-eight patients were below the age of 20 years and 108 were above. Age distribution is shown in Table 1. The most common causes of hydrocephalus were congenial anomaly and tumor in the age group below 20 years old, while subarachnoid hemorrhage and trauma was most common in the above 20 years old group (Table 2). Distribution of revision causes and time intervals between original shunt surgery and revision according to each complication are shown in Table 3. Infection and obstruction were the most common causes of revision, occurring in 46 (31.5%) and 38 (26.0%) cases, respectively. Fifty-one patients (34.9%) were operated on within 1 month after the original surgery. Thirty-one of these were due to infection and malposition of the proximal catheter. Thirty-six of the 46 infection cases were operated in the 6 months following the original VP shunt. Infection developed more frequently before 6 months after the original shunt while obstruction and fracture developed more after 6 months (p<0.05). Nine of 13 shunt catheter fractures developed more than 24 months after the VP shunt. Shunt catheter fractures were significantly higher in the younger patients (p=0.006) (Table 4). In addition, shunt catheter fracture was different between the age groups. Two of 8 fractured catheters in the younger group were due to calcification and degradation of shunt catheters with fibrous adhesion to surrounding tissue, which were not found in adults. All such fractures due to degradation and calcification of the catheter developed later than 9 years after shunt surgery.

Relationship between the cause of revision surgery and the time interval after initial ventriculoperitoneal shunt placement
DISCUSSION
Hydrocephalus is a common neurosurgical disease that develops via a variety of etiologies, including congenital anomaly, intracranial hemorrhage, infection, and tumor. Hydrocephalus can develop at any age, from prematurity to the very old. Even though a VP shunt is the main treatment modality for hydrocephalus, there can exist very different surgical environments, including patient age, etiologies, shunt materials used, and surgeon's experiences24). Special consideration should be paid concerning the patient's preoperative condition.
A VP shunt complication is a major obstacle in the management of hydrocephalus. Further, it is conceivable that the features of VP shunt complication can differ according to a patient's age and the etiology of the hydrocephalus. The incidence of complications following VP shunt placement is reported to be around 20 to 40%11023). However, Stone et al.34) reported 84.5% of their patients had required shunt revision on 15 year follow up of pediatric shunt surgeries. Stein and Guo32) reported the 5 year shunt survival rates in children and adults, estimated using mathematical model, were 49.4 and 60.2%, respectively. Even though patient deaths are greater in adults with shunt insertions, shunts in adults fail more slowly and tend to survive longer than those in children32). The incidence of shunt failure is higher in the first six months following the VP shunt23). The cause of shunt malfunction is different according to the time interval following VP shunt placement23). According to our series, infection developed more frequently within 6 months after the original shunt surgery, while obstruction and fracture occurred more commonly later.
Infection is one of the most common complications following shunt surgery. The rate of infection has constantly reduced since Choux et al.5) reported dramatic reduction through their meticulously concerned strategies. Recently, antibiotic-impregnated shunt catheters have been shown to reduce the infection rate significantly1526). It is noteworthy that Demetriades and Bassi8) reported a series of rifampicin resistant Staphylococcus epidermis infections and suggested the possibility for the development of new, rare, and resistant staphylococcus strains as a cause of VP shunt infection. One of the most common causes of early revision is malposition of the catheter, especially proximal malposition. Several authors proposed operative methods to introduce the proximal catheter more accurately. Crowley et al.7) used intraoperative ultrasound with significantly accurate insertion of the proximal catheter. Neuronavigation guidance provided longer shunt survival by accurate insertion of the proximal catheter, especially in patients with a small ventricle, such as slit ventricle syndrome, or benign intracranial hypertension1420). Laparoscopic insertion of a peritoneal catheter was also proposed for distal catheter insertion or revision42122273335). It is relatively difficult to introduce a peritoneal catheter in patients with a past history of abdominal surgery4). In our series, all the malposition cases were on the proximal side.
An adjustable pressure valve contributed to reducing the revision rate due to over-or under-drainage. Farahmand et al.10) reported a decreased 6-months failure rate in patients who had used an adjustable shunt valve. They also mentioned that frontal placement of a proximal catheter was associated with a lower rate of revision. However in young children, it is recommended that the proximal catheter should be inserted through the occipital or posterior parietal route.
Shunt fractures are relatively common complications of the VP shunt both in children and adults. Reddy et al.24) reported the shunt outcome in patient who passed 17 years of age and had undergone shunt surgery before 17 years. Overall the shunt failure rate was 82.9% and shunt complications were more frequent in the younger patients compared with adults. Forty eight among 105 revision patients were shunt disconnection, catheter leakage/break, shunt extrusion, migration, or catheter protrusion24). According to the authors, infection, obstruction and overdrainge were the main causes of shunt malfunction in adult transition24). Stone et al.34) reported shunt tubing break in 4% of their shunt malfunction on their more than 15 year follow up pediatric shunt patients. They did not mention the reason of tube break or the differences with that of adults. In our series, the shunt fracture was more frequent in the young age group and two out of 8 cases of shunt catheter fracture in patients younger than 20 years of age were related to catheter degradation associated with calcification and growth in patients3121631). These calcifications were found only in the younger group. Degradation associated with calcification of the catheter results in fibrosis and tight adhesion of the catheter to the surrounding soft tissue. It prevents the sliding of the catheter necessary to adjust to the growth of children. It is one of the unsolved problems involved with current catheter materials. Silastic is the commonly used material for the manufacture of the implantation devices. However, complications due to the material itself, such as hypersensitivity or tissue reaction, are still unsolved problems113036). Almost all literature about shunt calcification involves children. Boch et al.3) hypothesized the reason for the complications to be the relative elevation of serum phosphorus in children compared with adults, as in cardiac bioprosthetic valves613). The exact mechanism is not clear; however, microdefects of the catheter or barium impregnation could be factors in the development of calcification. Echizenya et al.9) proposed that the host's age and the duration of system implantation could be correlated with the incidence of mineralization. They reported the progressive change of the catheter with remarkable deterioration in systems implanted for more than 5 years, after they studied material from the removed shunt catheter9).
An important limitation of our study is that it is based on patients who received revision surgery during a designated time at our institution. To evaluate the long-term complication rate of shunt surgery, it is desirable that the study should be based on a strenuous long-term follow-up of all patients operated on during the designated time duration. However, it is very difficult and only possible at a highly sophisticated institute32). Because it was not possible at our hospital, we evaluated patients who had revision surgery during a relatively long time-duration. Because our hospital receives many hydrocephalus referrals and has an active pediatric neurosurgery department, we assumed the analysis of revision surgery cases during a 10-year time interval could indirectly represent a pattern of shunt complications.
CONCLUSION
We retrospectively analyzed shunt revision surgery performed in our hospital to elucidate the difference of the complication according to ages. The incidence of shunt catheter fracture was higher in younger patients. Degradation of the shunt catheter associated with surrounding tissue calcification could be one of the reasons of the difference in fracture rates.